Application of saringosterol
A technology of sargassum sterol and application, applied in the field of sargassum sterol as LXR agonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
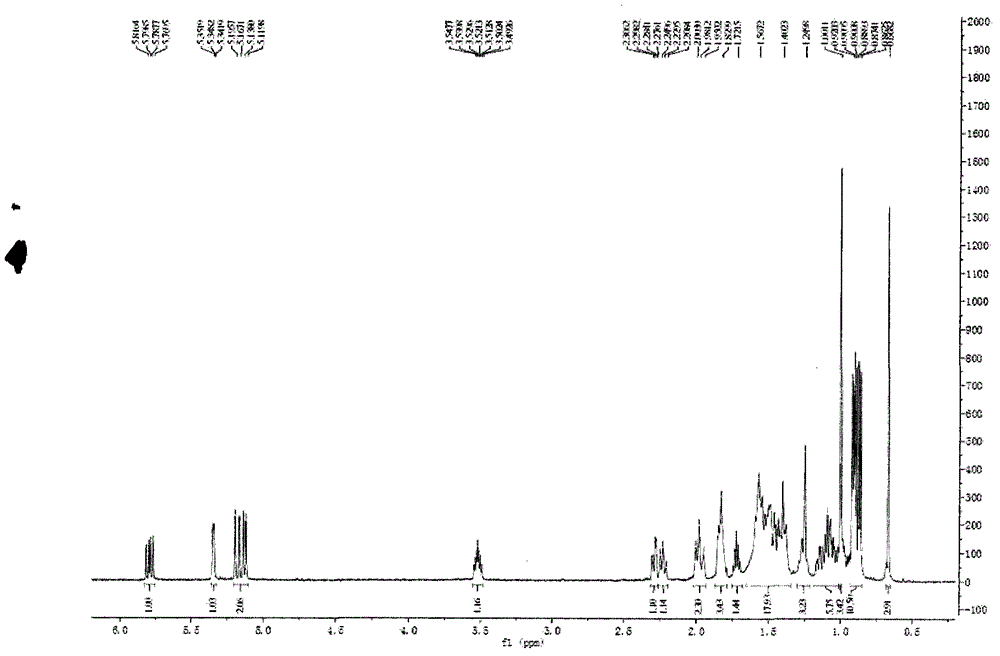
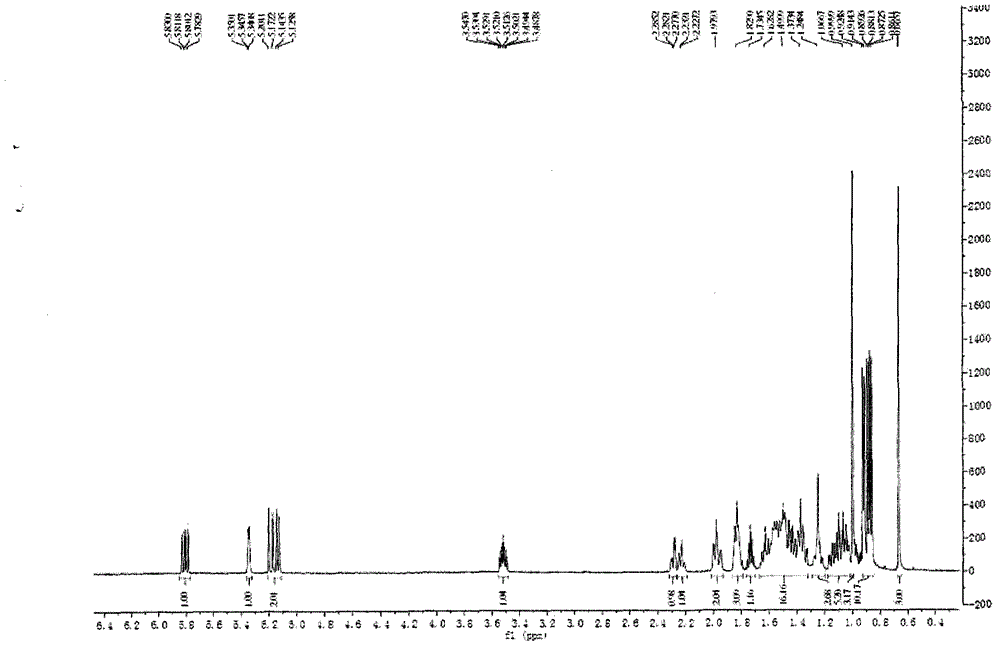
[0026] Example 1: Extraction and Separation and Purification of 24(S)-Sargasso Sterol
[0027] Take 500 g of Sargassum sargassum, and use 10 times the volume of dichloromethane-methanol with a volume fraction ratio of 2:1 to reflux for 2 hours, and repeat 3 times. The extracts were combined, filtered, and concentrated to dry solvent to obtain 31.5 g of extract.
[0028]After the extract (31.5g) was dissolved in dichloromethane, mix the sample with 41g 200-300 mesh silica gel H (product of Qingdao Ocean Chemical Group Co., Ltd.), remove the solvent under reduced pressure, use silica gel column chromatography, and use cyclohexane-dichloro Methane and dichloromethane-methanol were used as solvents for gradient elution, and the eluate of dichloromethane-methanol (v / v 99:1) was subjected to Sephadex LH-20 gel column chromatography (dichloromethane-methanol 1 : 1 elution), the resulting fraction was recrystallized from dichloromethane-methanol to obtain sargassum sterol. Sargasso ...
Embodiment 2
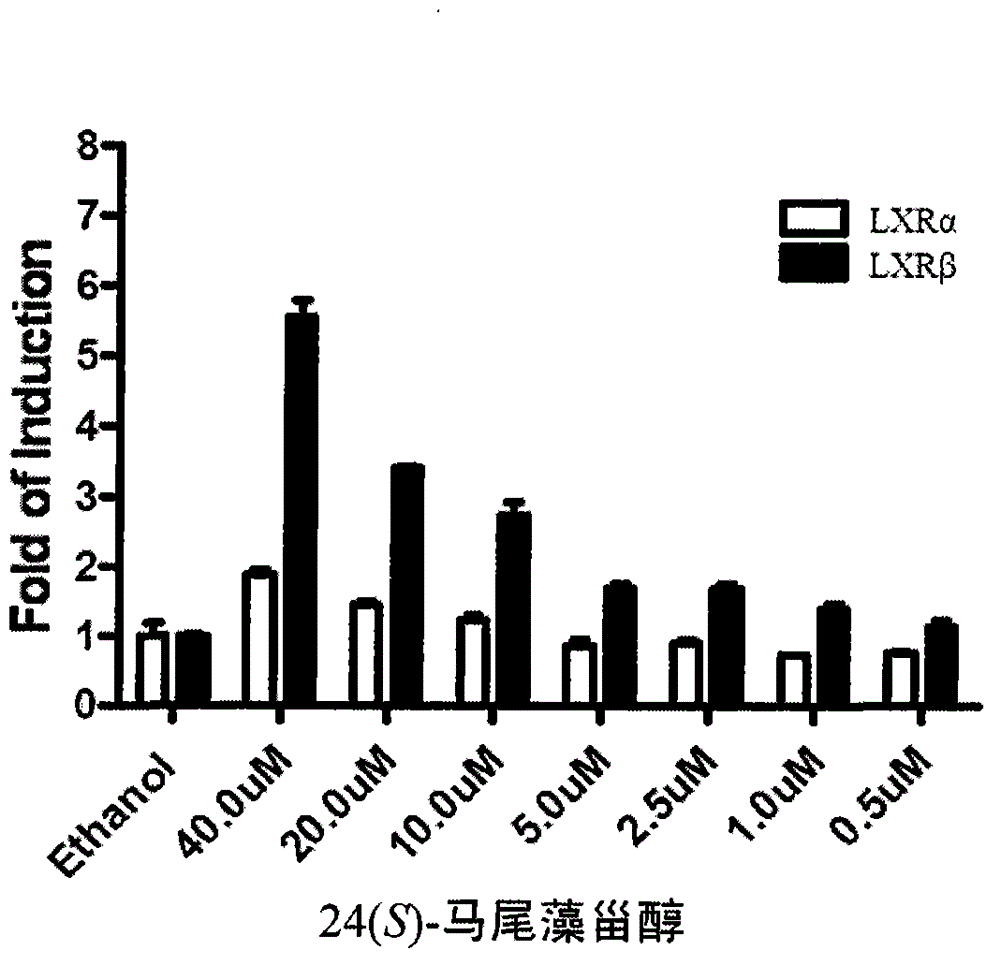
[0032] Example 2: Testing of LXRβ Activation
[0033] Transcriptional activation of LXRβ was detected using a dual-luciferase reporter gene assay. 293T cells were inoculated in 48-well plates with DMEM medium (10% FBS, without antibiotics). After 8-12 hours, the cells grew to about 60%. The medium was not changed, and the plasmid was directly transfected according to the instructions of lipo2000. The total amount of plasmids was 0.075 μg / well (0.05 μg TK-luc containing UAS element, 0.005 μg internal control pRL-TK and 0.02 μg Gal4 fusion protein expression plasmid GAL4-LXRα / β containing LXRα / β LBD domain). The amount of lipo2000 used was 2.5 times the mass of the transfected plasmid (2.5*0.075 μl=0.1875ul / well). Plasmid and lipo2000 were mixed in 25μl / well optim medium in advance. Add phytosterol samples 12 hours after transfection, and detect Luciferase activity 24 hours later. The results showed that at the concentration of 100 μM, the activation folds of sargassum sterol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com