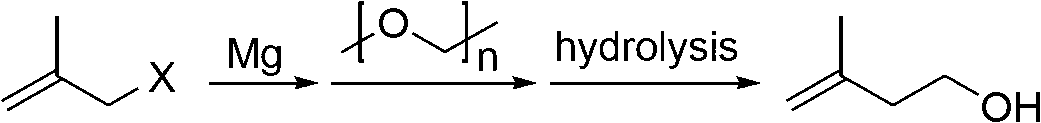Method for preparing 3-methyl-3-buten-1-ol
A technology of butene and methyl is applied in the field of preparation of water reducing agent intermediates, which can solve the problems of difficulty in storage and transportation of raw materials, harsh process conditions, etc., and achieves the effect of simple process and easy storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
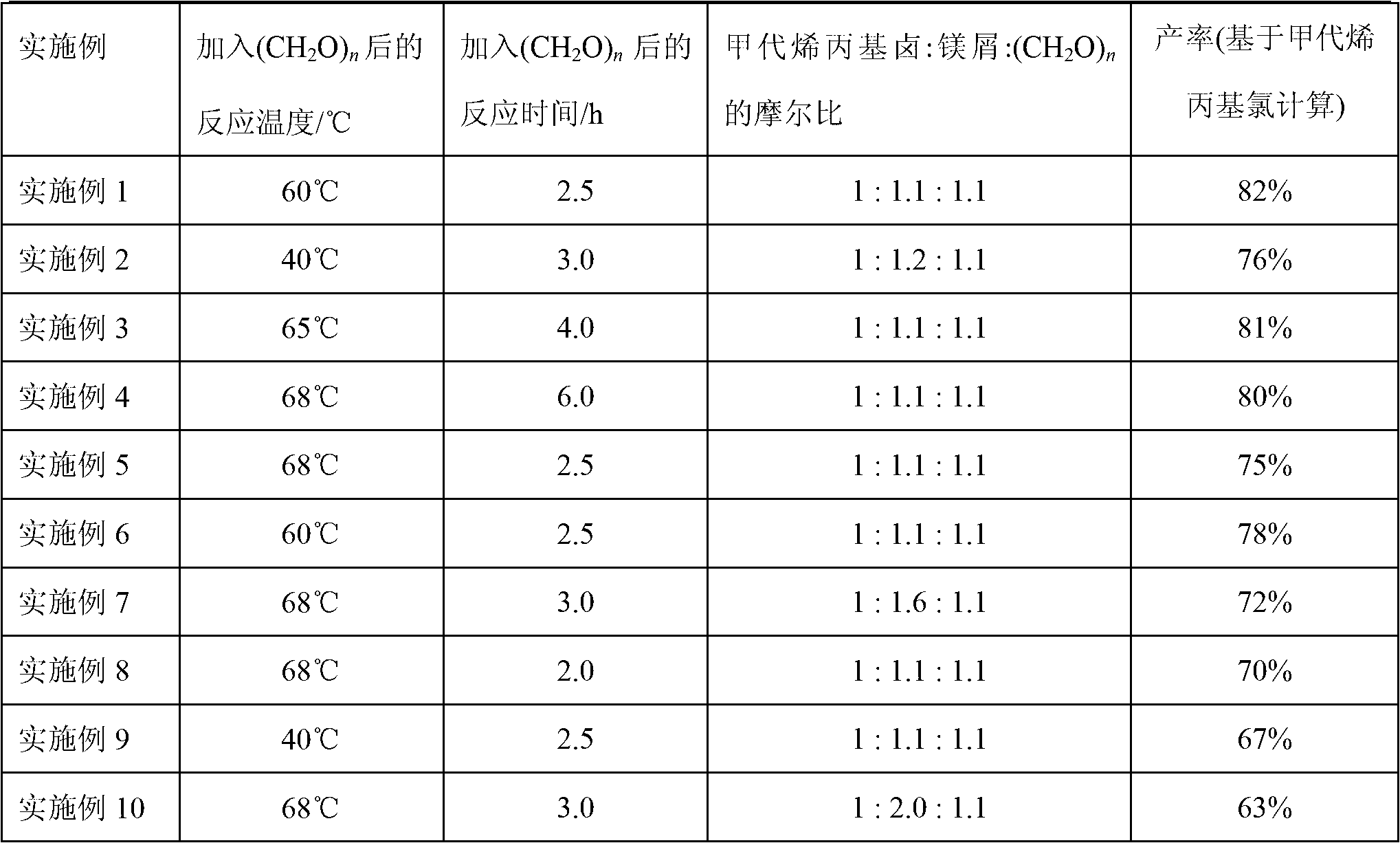
[0026] Add 80mL of anhydrous tetrahydrofuran to a 500mL three-necked flask equipped with magnetic stirring, constant pressure dropping funnel and reflux condenser, then add 7.84g (0.327mol) of polished magnesium chips, and then add 0.254g (0.001mol) Iodine, blow nitrogen, add 0.327g (0.003mol) ethyl bromide dropwise at room temperature, wait until the color of the mixture becomes lighter and the reaction starts, add 100mL tetrahydrofuran and 30mL (0.297mol) methallyl Chlorine solution, the temperature of the reaction mixture is controlled below 40°C during the dropwise addition. After the dropwise addition, continue to react for 2h, then add 9.36g (0.104mol) of paraformaldehyde (n=3), raise the temperature to 60°C, react for 2.5h, then cool to room temperature, add dropwise 200mL of saturated ammonium chloride solution to three ports The product was hydrolyzed in a flask, the obtained aqueous layer was extracted 3 times with anhydrous ether, the obtained organic phase was drie...
Embodiment 2
[0029] Add 150mL of anhydrous diethyl ether to a 1000mL three-necked flask equipped with magnetic stirring, constant pressure dropping funnel and reflux condenser, then add 14.11g (0.588mol) of polished magnesium chips, and then add 0.762g (0.003mol) Iodine, blow argon, drop 0.436g (0.004mol) ethyl bromide at room temperature, wait until the color of the mixed solution becomes lighter, that is, after the reaction starts, add 150mL ether and 50mL (0.511mol) methallyl dropwise The temperature of the reaction mixture is controlled below 40°C during the dropwise addition. After the dropwise addition, continue to react for 1 hour, then add 16.86g (0.562mol) formaldehyde (n=1), raise the temperature to 40°C, react for 3 hours, cool to room temperature, add dropwise 250mL saturated ammonium chloride solution to the three-necked flask to test the product Carry out hydrolysis, the obtained aqueous layer is extracted 3 times with anhydrous ether, the obtained organic phase is dried with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com