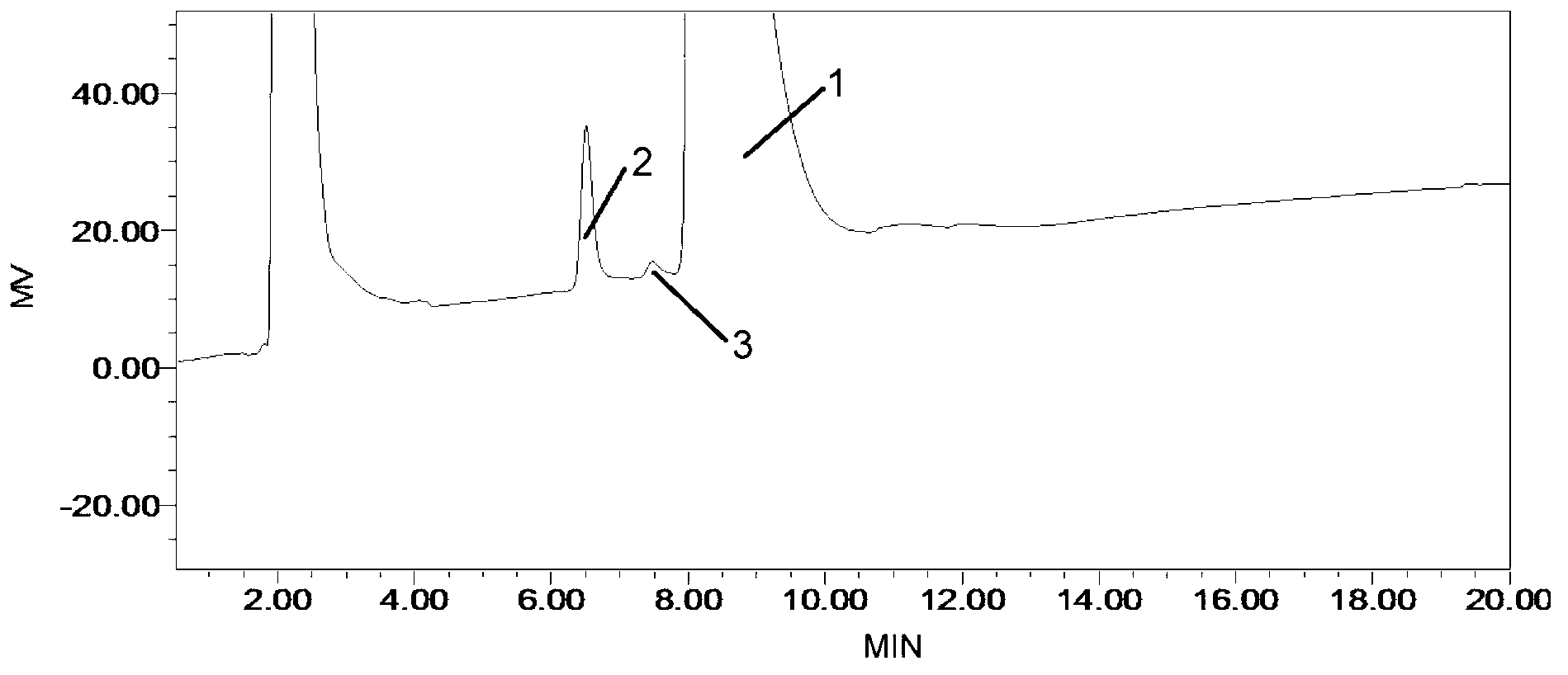
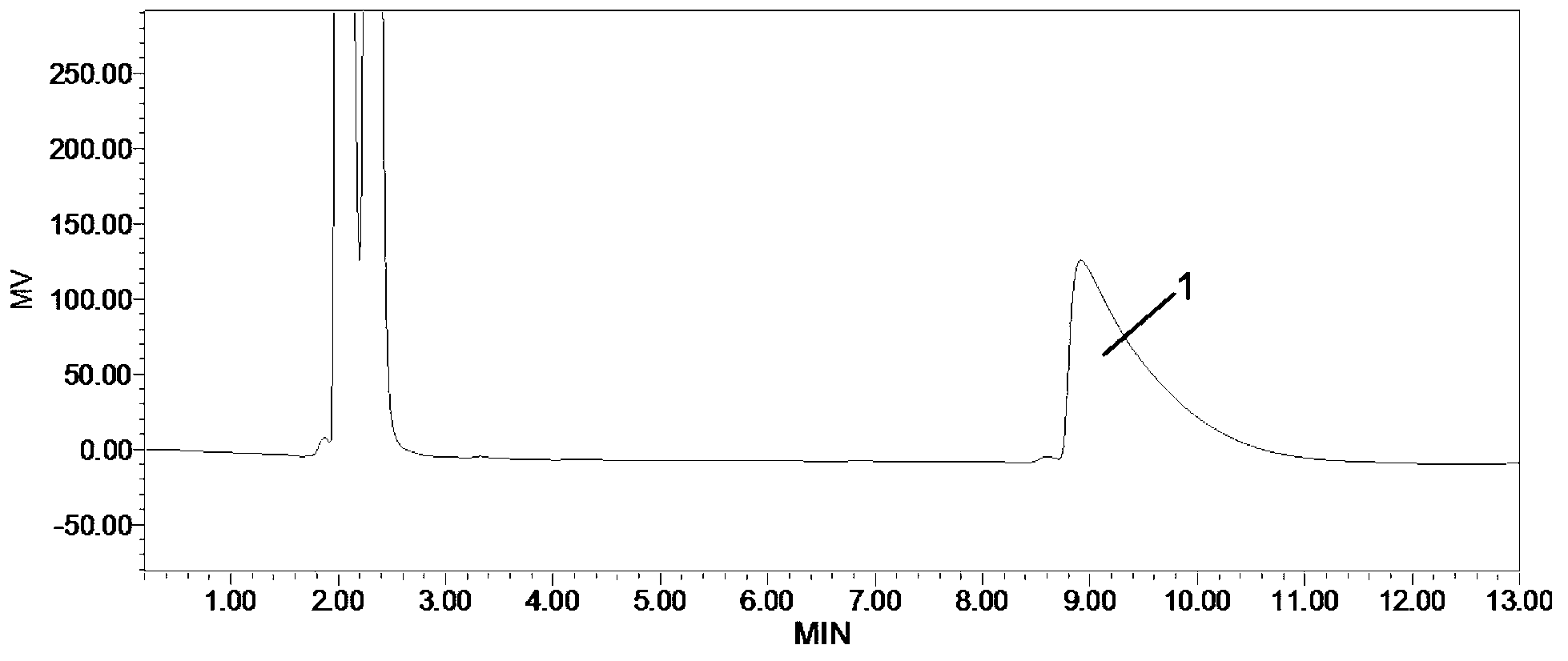
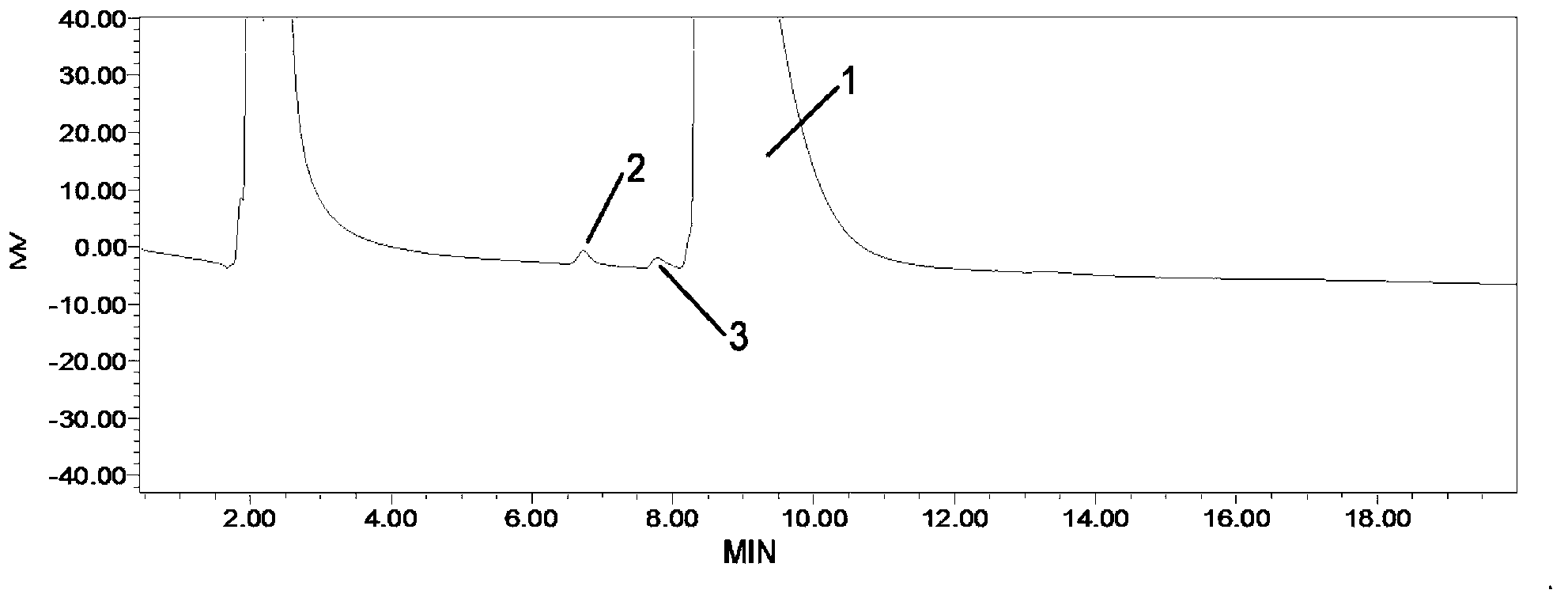
Method for detecting related substances in fosfomycin sodium by high-performance liquid chromatography
A high-performance liquid chromatography and detection technology of fosfomycin sodium, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of unrecorded and other problems, and achieve the effects of mild detection conditions, simple preparation, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Chromatographic conditions
[0032] Chromatographic column: Zorbax-NH2 (4.6×250mm, 5μm)
[0033] Detector: RID (Refractive Index Detector)
[0034] Detector temperature: 35°C
[0035] Wherein, the chromatographic column Zorbax-NH2 is an amino-propylsilane bonded silica gel column of Agilent Company.
[0036] Preparation of the mobile phase: Accurately weigh 10.89 g of potassium dihydrogen phosphate, dissolve in 1000 ml of purified water, filter, and sonicate to obtain a 0.08 mol / l potassium dihydrogen phosphate solution.
[0037] (2) Preparation of reference solution
[0038] Dissolve 0.6g fosfomycin sodium standard substance with mobile phase, and dilute to 5.0ml with mobile phase, obtain the reference substance solution that every 1ml contains 0.12g fosfomycin sodium standard substance.
[0039] (3) Refer to the preparation of solution 1
[0040] (a) Wet 0.3 g of the test sample with 60 μl of water, and heat in an oven at 60°C for 24 hours. Dissolve the resi...
Embodiment 2
[0065] (1) Chromatographic conditions
[0066] Chromatographic column: Zorbax-NH2 (4.6×250mm, 5μm)
[0067] Detector: RID (Refractive Index Detector)
[0068] Detector temperature: 30°C
[0069] Wherein, the chromatographic column Zorbax-NH2 is an amino-propylsilane bonded silica gel column of Agilent Company.
[0070] Preparation of mobile phase: Accurately weigh 6.80 g of potassium dihydrogen phosphate, dissolve in 1000 ml of purified water, filter, and sonicate to obtain a 0.05 mol / l potassium dihydrogen phosphate solution.
[0071] (2) Preparation of reference solution
[0072] Dissolve 0.3g fosfomycin sodium standard substance with mobile phase, and dilute to 5.0ml with mobile phase, obtain the reference substance solution that every 1ml contains 0.06g fosfomycin sodium standard substance.
[0073] (3) Refer to the preparation of solution 1
[0074] (a) Wet 0.2 g of the test sample with 30 μl of water, and heat in an oven at 50°C for 30 hours. Dissolve the residue w...
Embodiment 3
[0091] (1) Chromatographic conditions
[0092] Chromatographic column: Zorbax-NH2 (4.6×250mm, 5μm)
[0093] Detector: RID (Refractive Index Detector)
[0094] Detector temperature: 40°C
[0095] Wherein, the chromatographic column Zorbax-NH2 is an amino-propylsilane bonded silica gel column of Agilent Company.
[0096] Preparation of mobile phase: Accurately weigh 16.33 g of potassium dihydrogen phosphate, dissolve in 1000 ml of purified water, filter, and sonicate to obtain a 0.12 mol / l potassium dihydrogen phosphate solution.
[0097] (2) Preparation of reference solution
[0098] Dissolve 1.2g fosfomycin sodium standard substance with mobile phase, and dilute to 5.0ml with mobile phase, obtain the reference substance solution that every 1ml contains 0.24g fosfomycin sodium standard substance.
[0099] (3) Refer to the preparation of solution 1
[0100] (a) Wet 0.4 g of the test sample with 90 μl of water and heat it in an oven at 70°C for 16 hours. Dissolve the residu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com