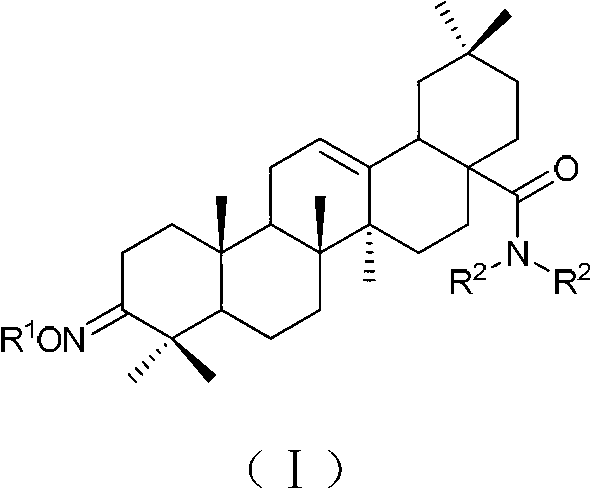
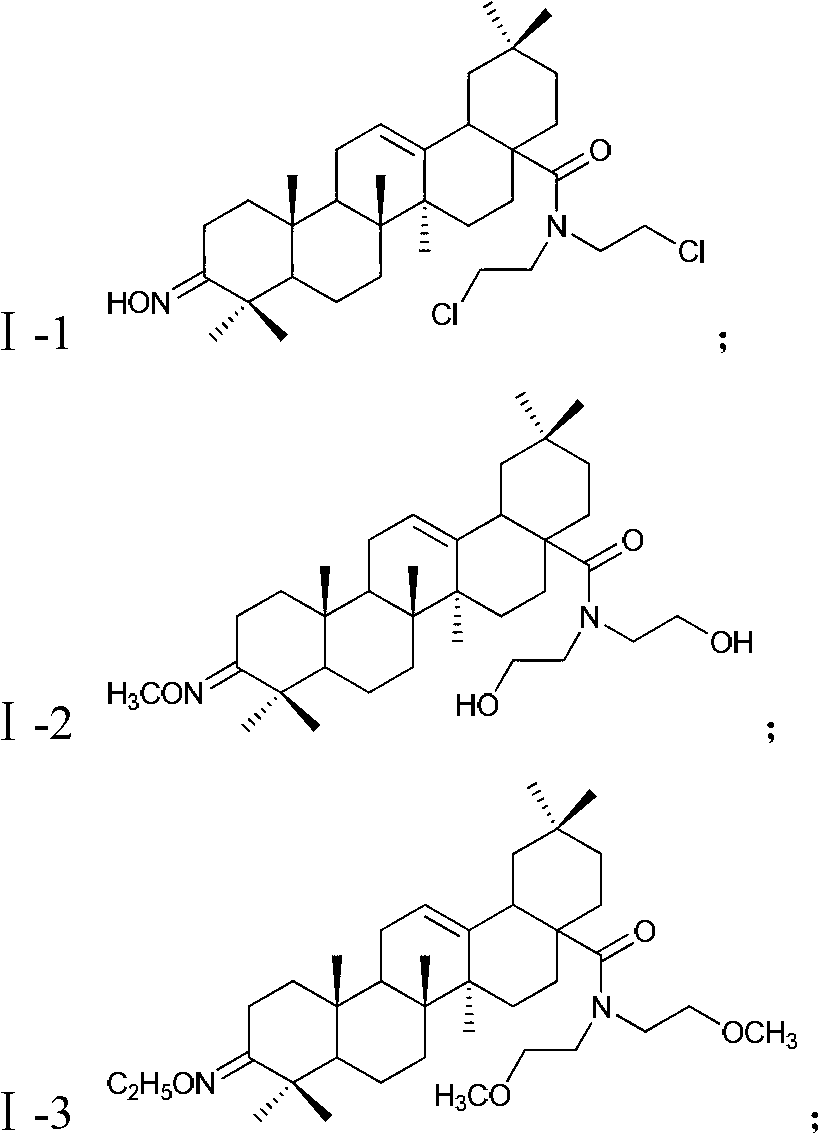
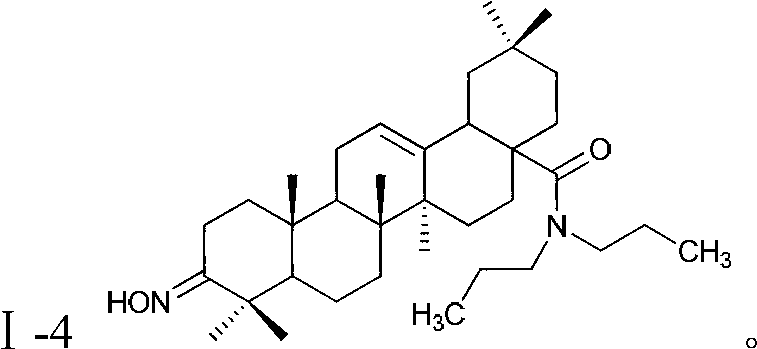
Oleanolic acid derivative with function of resisting malignant tumor, as well as preparation method and applications of oleanolic acid derivative
A compound and straight-chain technology, applied in the field of medicine, can solve problems such as tissue and organ damage, death, and lower quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Reference Example 1: Synthesis of Intermediate II
[0064]
[0065] Dissolve 4.57g (0.01mol) of oleanolic acid in 25mL of dichloromethane, add dropwise 30mL of 5.40g (0.025mol) of PCC in dichloromethane, then add the same amount of diatomaceous earth as PCC, and stir the reaction at room temperature , TLC to monitor the progress of the reaction. Filter after completion of the reaction, wash the filtrate with 3×50mL water, separate the dichloromethane layer, fully dry it with anhydrous sodium sulfate, filter, evaporate the dichloromethane under reduced pressure, and separate the column [mobile phase: v (petroleum ether): v(ethyl acetate)=4:1] to obtain 2.77 g of white solid product (HPLC: 99.3%), yield 60.8%.
Embodiment 2
[0066] Reference Example 2: Synthesis of Intermediate Ⅲ-1
[0067]
[0068] Add 4.55g (0.01mol) of intermediate II to a reaction flask equipped with a stirring, condenser and thermometer, dissolve it with 30mL of anhydrous methanol, and add 10mL of pyridine while stirring. Add 2.78g (0.04mol) of hydroxylammonium hydrochloride to the reaction system in batches. After the addition was complete, the reaction was continued at 50°C for 3.5h (the plate showed that the reaction was complete). Evaporate anhydrous methanol to dryness, wash the reaction solution with 3 × 30mL water, extract with dichloromethane, fully dry over anhydrous sodium sulfate, filter, and evaporate dichloromethane under reduced pressure to obtain 2.0 g of white solid (HPLC: 94.5% ), the yield was 43.5%.
Embodiment 3
[0069] Reference Example 3: Synthesis of Intermediate Ⅲ-2
[0070]
[0071] Add 4.55 g (0.01 mol) of intermediate II to a reaction flask equipped with stirring, a condenser, and a thermometer, dissolve it with 25 mL of acetone, and add 2.24 g of potassium hydroxide while stirring. 3.34g (0.04mol) methoxyamine hydrochloride was added to the reaction system in batches. After the addition was complete, the reaction was continued at 40°C for 2h (the plate showed that the reaction was complete). Evaporate the acetone to dryness, wash the reaction liquid with 3×30mL water, extract with dichloromethane, fully dry with anhydrous sodium sulfate, filter, evaporate dichloromethane under reduced pressure to obtain 3.0g of white solid (HPLC: 96.7%), Yield 62.5%.
[0072] Reference Example 4: Synthesis of Intermediate Ⅲ-3
[0073]
[0074] Add 4.55 g (0.01 mol) of intermediate II to a reaction flask equipped with stirring, a condenser, and a thermometer, dissolve it with 40 mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com