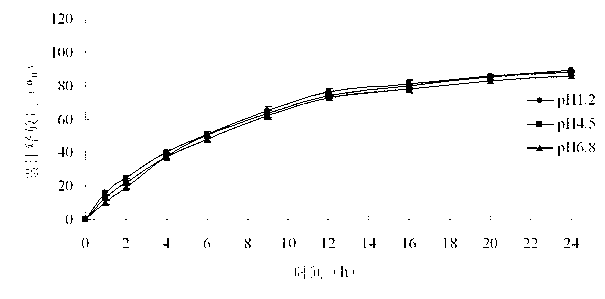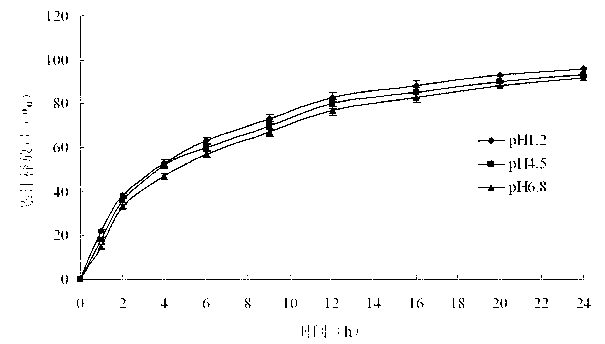Pramipexole dihydrochloride slow-release tablet with high content uniformity and preparation method thereof
A technology for pramipexole hydrochloride and sustained-release tablets, which is applied in the field of pramipexole hydrochloride sustained-release tablets and its preparation, and can solve unqualified content uniformity and release standard deviation, increased research and development costs, and sample quality differences, etc. problems, to avoid incomplete drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]The pramipexole hydrochloride sustained-release tablets shown in Table 1 were prepared.
[0040] Table 1. The pramipexole hydrochloride sustained-release tablets of embodiment 1
[0041]
[0042] Remarks: Ethanol water solution is used in the granulation process, it is removed by drying and does not exist in the final product.
[0043] Preparation method: dissolve the pramipexole hydrochloride and hypromellose of prescription quantity in 40% ethanol aqueous solution, and use it as a drug-containing adhesive for subsequent use; place hypromellose and microcrystalline cellulose in a fluidized bed, After mixing for 3-5 minutes, spray the drug-containing binder into the bed for granulation. After spraying, dry; add magnesium stearate, mix evenly, and press into tablets. The hardness range is controlled at 5-10kg.
Embodiment 2
[0045] Prepare pramipexole hydrochloride sustained-release tablets shown in Table 2.
[0046] Table 2. The pramipexole hydrochloride sustained-release tablets of embodiment 2
[0047]
[0048] Remarks: Ethanol water solution is used in the granulation process, it is removed by drying and does not exist in the final product.
[0049] Preparation method: Dissolve the prescribed amount of pramipexole hydrochloride and hypromellose in 70% ethanol aqueous solution, and use it as a drug-containing adhesive for later use; place hypromellose and microcrystalline cellulose in a high-shear wet process In the granulator, mix evenly, spray the drug-containing binder into the high-shear wet granulator at a constant speed to granulate, and dry; add magnesium stearate, mix evenly, and press into tablets. The hardness range is controlled at 6-12kg .
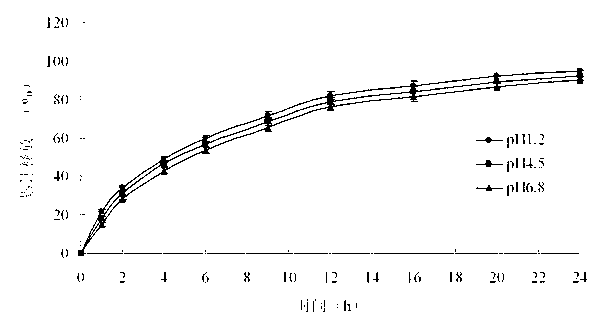
Embodiment 3
[0051] Prepare pramipexole hydrochloride sustained-release tablets shown in Table 3.
[0052] Table 3. The pramipexole hydrochloride sustained-release tablets of embodiment 3
[0053]
[0054] Remarks: Ethanol water solution is used in the granulation process, it is removed by drying and does not exist in the final product.
[0055] Preparation method: dissolve pramipexole hydrochloride and hypromellose of prescribed quantity in 60% ethanol aqueous solution, and use it as a drug-containing adhesive for subsequent use; place hypromellose and microcrystalline cellulose in a fluidized bed, After mixing for 3-5 minutes, spray the drug-containing binder into the bed for granulation. After spraying, dry; add magnesium stearate, mix evenly, and press into tablets. The hardness range is controlled at 5-10kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com