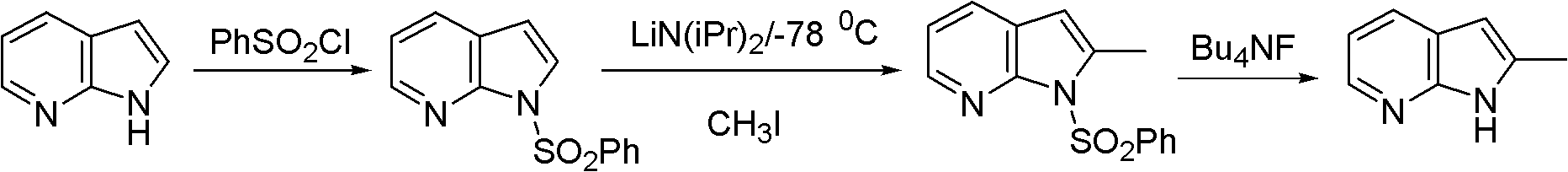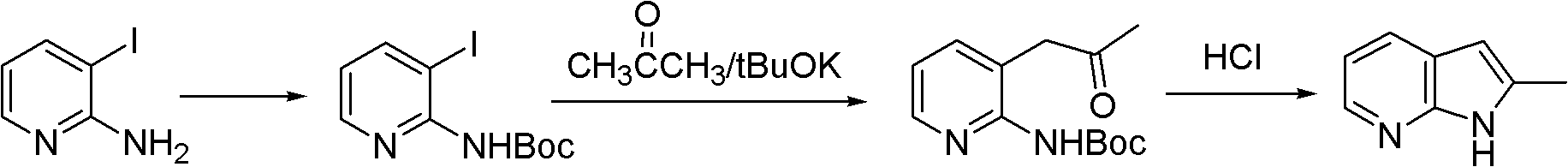Preparation method of 2-methyl-7-azaindole
An azaindole and methyl technology, which is applied in the field of preparation of 2-methyl-7-azaindole, can solve the problems of expensive raw material reagents, intense reaction process, harsh production conditions and the like, and achieves low cost, The effect of easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of 2-methyl-7-azaindole
[0030] (1) 2-amino-3-picoline is acylated with acetic anhydride to generate 2-acetylamino-3-picoline
[0031] In a 100L reactor, add 10Kg of 2-amino-3-picoline, 40Kg of toluene and 15Kg of acetic anhydride, slowly raise the temperature to 60°C for reaction, react for 2 hours, and monitor and control the raw material <1% by HPLC; cool to 40°C, concentrate under reduced pressure Recover toluene, transfer the residue to a 500L reaction kettle, add 100Kg water to quench, adjust the pH to 10 with sodium carbonate, extract 50Kg×3 with dichloromethane, combine the extracts, wash with saturated saline, dry over anhydrous sodium sulfate, and filter; Concentrate under reduced pressure to dryness (dichloromethane is recovered) to obtain 12.7Kg (92%) of crude product. The crude product can be directly put into the next step of cyclization reaction. Or recrystallize the crude product with 38.1Kg of isopropanol, heat up and reflux to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com