Method for chiral separation of various side chain protected amino acids
A chiral separation and side chain protection technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of unstable chiral chromatographic column and cumbersome mobile phase system, and achieve good chiral separation effect. , Wide range of use, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
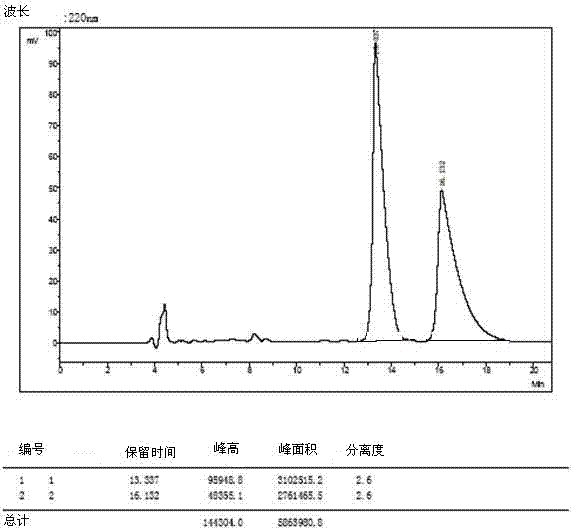
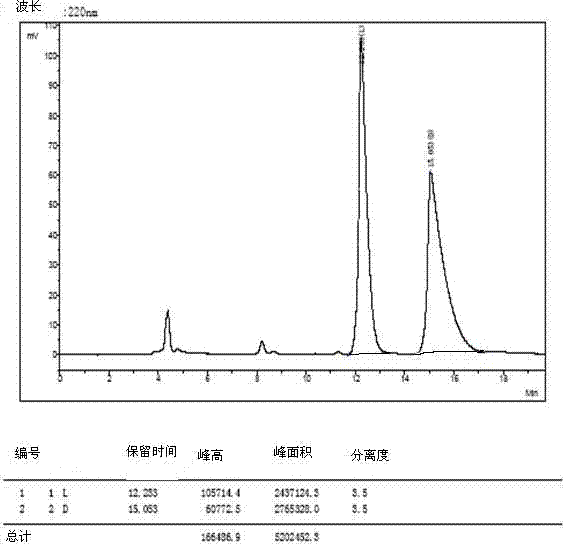
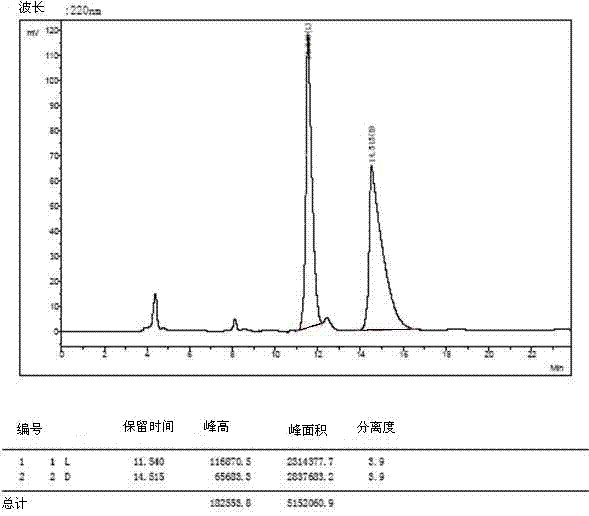
[0035] The 4 racemates were dissolved and filtered:
[0036] 1) Weigh 3mg of O-tert-butyl-L-tyrosine and 3mg of O-tert-butyl-D-tyrosine and dissolve them in a 10ml volumetric flask (that is, prepare 0.6mg / ml concentration of O- tert-butyl-DL-tyrosine), dissolved in a 40:60 volume ratio of methanol and aqueous solution, ultrasonically treated, until the sample is completely clear, add the solution to the mark, filter with a 0.45 μm organic phase filter head, and take the filtrate for use .
[0037] 2) The other three amino acid derivatives were dissolved, and 5mg of L-type and 5mg of D-type samples were weighed and placed in 1ml sample tubes (that is, DL-glutamic acid-5-tert-butyl with a concentration of 10mg / ml was prepared ester, O-tert-butyl-DL-serine, DL-aspartic acid-4-tert-butyl ester), dissolved in methanol to aqueous solution with a volume ratio of 40:60, sonicated, until the sample was completely clarified, washed with 0.45 μm Filter the organic phase with a filter h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com