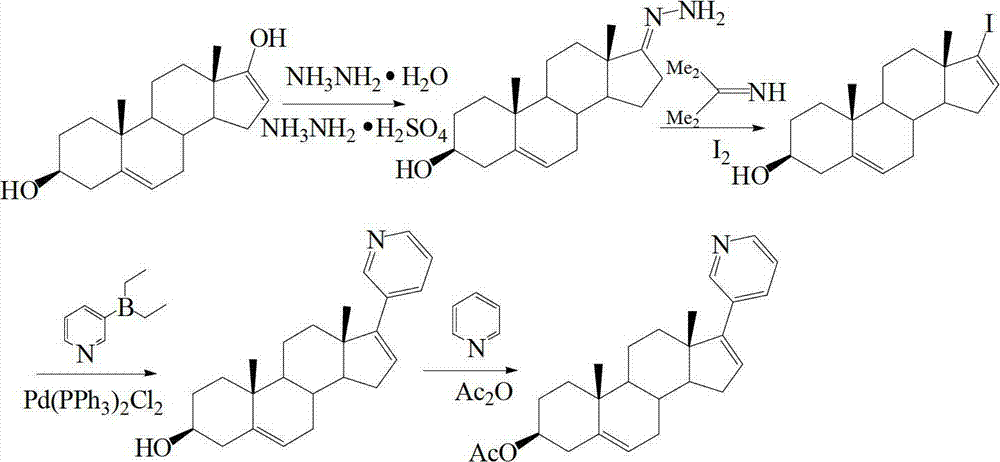
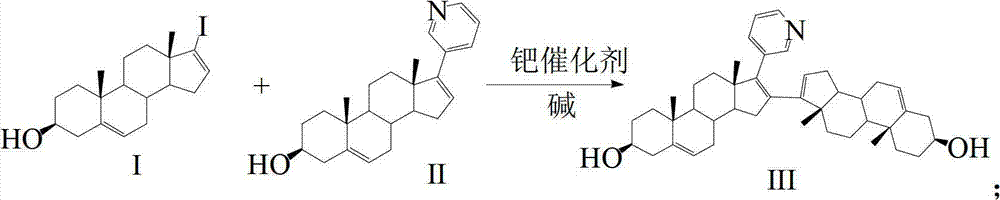
Preparation and detection method of abiraterone Acetate dimer compound
A technology of abiraterone acetate and a detection method, which is applied in the field of preparation and detection of abiraterone acetate dimer compounds, can solve complex preparation methods, low purity and yield of abiraterone acetate dimer compounds, and environmental pollution and other problems, to achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Step 101, Synthesis of Compound III
[0047] Under nitrogen protection, 3.98 g of 10 mmol of compound I: 3β-hydroxy-17-iodo-androst-5,16-diene and 3.49 g of 10 mmol of compound II: 17-(3-pyridyl) androst-5,16 -diene-3β-alcohol is placed in the reactor, after adding 100ml of dioxane to dissolve, add 0.69 grams of Pd (PPh 3 ) 4 , 3.18 grams of 30mmol sodium carbonate, refluxed for 26h, reacted completely, cooled to room temperature, filtered, concentrated filtrate, added 30ml of water, extracted with ethyl acetate, concentrated filtrate, obtained 5.58 grams of 9mmol compound III: 3β-hydroxyl-16-(3β -Hydroxyandrost-5,16-dien-17-yl)-17-(3-pyridyl)androst-5,16-diene as crude white solid, yield 90.0%.
[0048] Step 102, synthesis of abiraterone acetate dimer compound
[0049] 5.58 grams of 9 mmol compound III obtained in step 101: 3β-hydroxyl-16-(3β-hydroxyandrosta-5,16-dien-17-yl)-17-(3-pyridyl)androsta-5, Put the crude product of 16-diene in the reactor, add 15ml of pyr...
Embodiment 2
[0054] Step 201, Synthesis of Compound III
[0055] Under nitrogen protection, 7.96 g of 20 mmol of compound I: 3β-hydroxy-17-iodo-androst-5,16-diene and 3.49 g of 10 mmol of compound II: 17-(3-pyridyl) androst-5,16 - Diene-3β-alcohol is placed in the reactor, add 100ml THF to dissolve, then add 0.5g Pd(OAc) into the reactor 2 , 4.14 grams of 30mmol potassium carbonate, refluxed for 26h, the reaction was complete, cooled to room temperature, filtered, concentrated the filtrate, added 30ml of water, extracted with dichloromethane, concentrated the filtrate to obtain 5.2 grams of compound III: 3β-hydroxyl-16-(3β- Hydroxyandrost-5,16-dien-17-yl)-17-(3-pyridyl)androst-5,16-diene as crude white solid, yield 83.9%.
[0056] Step 202, synthesizing abiraterone acetate dimer compound
[0057] 5.2 grams of 8.4 mmol of compound III obtained in step 201: 3β-hydroxyl-16-(3β-hydroxyandrost-5,16-dien-17-yl)-17-(3-pyridyl)androsta- Put the crude product of 5,16-diene in a reactor, add 15ml...
Embodiment 3
[0062] Step 301, Synthesis of Compound III
[0063] Under nitrogen protection, 11.94 g of 30 mmol of compound I: 3β-hydroxy-17-iodo-androst-5,16-diene and 3.49 g of 10 mmol of compound II: 17-(3-pyridyl) androst-5,16 -diene-3β-alcohol is placed in the reactor, after adding 100ml of acetonitrile to dissolve, add 0.8 g of PdCl to the reactor 2 (MeCN) 2, 6.5 grams of 20mmol cesium carbonate, refluxed for 20h, the reaction was complete, cooled to room temperature, filtered, the filtrate was concentrated, 30ml of water was added, extracted with chloroform, and the filtrate was concentrated to obtain 5.3 grams of compound III: 3β-hydroxyl-16-(3β- Hydroxyandrost-5,16-dien-17-yl)-17-(3-pyridyl)androst-5,16-diene as crude white solid, yield 85.5%.
[0064] Step 302, Synthesizing Abiraterone Acetate Dimer Compound
[0065] 5.3 grams of 8.6 mmol of compound III obtained in step 301: 3β-hydroxyl-16-(3β-hydroxyandrost-5,16-diene-17-yl)-17-(3-pyridyl)androsta- Put the crude product of 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com