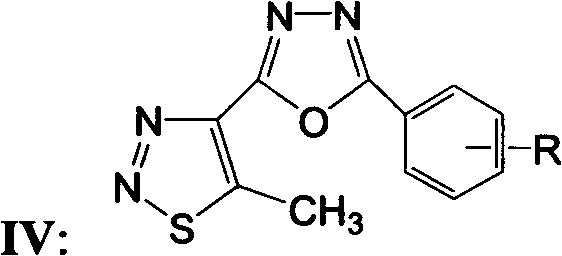
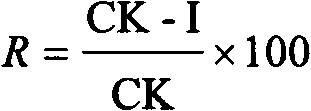5-methyl-1,2,3-thiadiazole-1,3,4-oxadiazole derivatives, and preparation method and application thereof
A technology of thiadiazole linkage and oxadiazole, applied in the field of 5-methyl-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis and structure identification of intermediate 5-methyl-1,2,3-thiadiazole-4-bishydrazide compound III:
[0032] In a 50 milliliter round bottom flask, add 3.15 millimoles of 5-methyl-1,2,3-thiadiazole-4-formylhydrazide, 6.3 millimoles of substituted benzoic acid, 6.3 millimoles of phosphorus pentachloride, 10 milliliters of benzene was heated to reflux for 3 hours. After the reaction was monitored by thin-layer chromatography, the reaction system was poured into ice water, and the pH value was adjusted to 7.0 with sodium bicarbonate, and extracted 3 times with ethyl acetate (20 milliliters × 3 ), the combined organic layers were dried with anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain compound III, then recrystallized with ethanol, calculated the yield with the pure product obtained, measured the melting point and 1 H NMR, the amount of synthetic compound III is enlarged or reduced according to the corresponding rat...
Embodiment 2
[0034] Synthesis and structure identification of 5-methyl-1,2,3-thiadiazole bi-1,3,4-oxadiazole derivative IV:
[0035] method one:
[0036] Add 6.3 mmoles of 5-methyl-1,2,3-thiadiazole-4-carboxylhydrazide, 6.3 mmoles of substituted benzoic acid, and 10 ml of phosphorus oxychloride into a 50 ml round bottom flask, and heat to reflux for 6 hours , after the completion of the reaction monitored by thin-layer chromatography, the reaction system was poured into ice water, the pH ester was adjusted to 7.0 with sodium carbonate, and then extracted 3 times with ethyl acetate (20ml×3), the organic layers were combined, and washed with anhydrous sulfuric acid The sodium was dried, the solvent was distilled off under reduced pressure, and the residue was separated by column chromatography with an eluent volume ratio of sherwood oil: ethyl acetate 2: 1 to obtain compound IV. Calculate the yield with the resulting pure product, measure the melting point and 1 H NMR, the amount of synthe...
Embodiment 3
[0040] The antibacterial or bactericidal activity of 5-methyl-1,2,3-thiadiazole 1,3-4-oxadiazole derivative IV of the present invention:
[0041] The names and codes of the common phytopathogenic fungi tested in the present invention include AS: Alternaria solani; BC: Botrytis cinerea; CA: Cercospora arachidicola; GZ: Wheat red Gibberella zeae; PI: Phytophthora infestans (Mont.) de Bary; PP: Physalosporapiricola; PS: Pelicularia sasakii; RC: Cereal silk Rhizoctonia cerealis; SS: Sclerotinia sclerotiorum, these strains are very representative and can represent most of the pathogenic bacteria species that occur in the field in agricultural production. The results of the bacterial growth rate method are shown in Table 2, and Table 2 shows that at 50 micrograms per milliliter, all compounds synthesized by the present invention have different degrees of bactericidal activity, TN-OXZ2, TN-OXZ4, TN-OXZ6, TN- The bactericidal activity of OXZ7 and TN-OXZ9 to CA was higher than 40%; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com