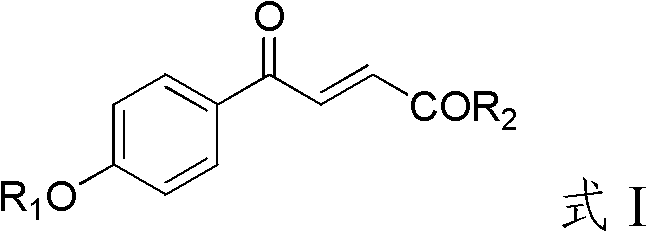
4-hydroxybenzoylacrylic acid derivative, preparation method thereof and application
A technology of hydroxybenzoyl acrylic acid and derivatives, which is applied in the field of 4-hydroxybenzoyl acrylic acid derivatives, can solve the problems of lack of treatment means and protection measures, and achieve good antibacterial effect, simple preparation method, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
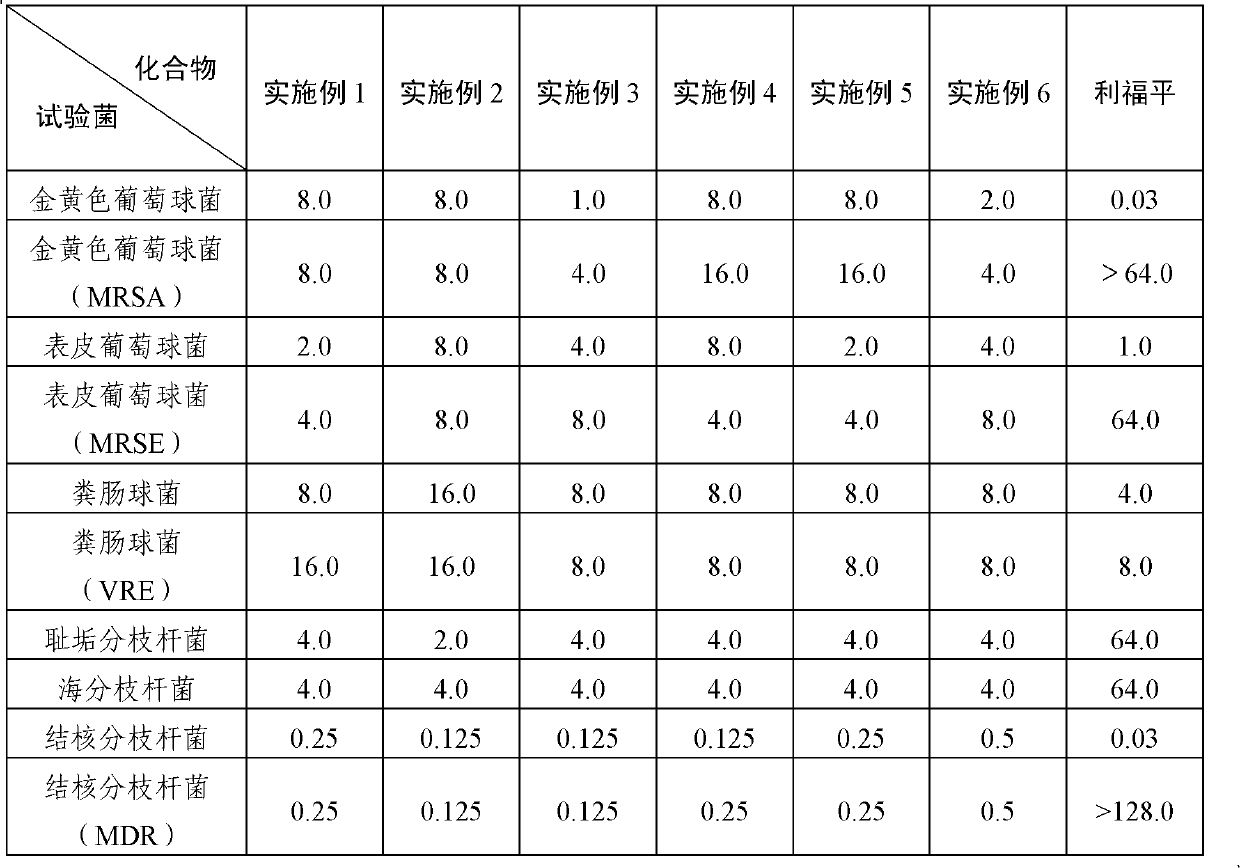
Examples
Embodiment 14
[0027] The preparation of embodiment 14-methyl methoxybenzoyl acrylate
[0028] (a) Preparation of 4-methoxybenzoylacrylic acid
[0029] Add 10g of 4-hydroxybenzoylacrylic acid (52.04mmol) and 10.77g of potassium carbonate (78.06mmol) into 100mL of acetone, stir and react at 25°C for 0.5 hours, then add 8.86g of methyl iodide (62.44mmol) dropwise, After the addition, the reaction solution was heated to 56°C for reflux reaction for 4 hours; after the reaction was completed, the acetone was removed by rotary evaporation under reduced pressure, and the residual solution was poured into 100 mL of ice water to precipitate a white solid, which was dried in vacuo to obtain 10.19 g of a white powdery solid, which was collected The rate is 95%. The test results are as follows:
[0030] 1 H NMR (400MHz, DMSO-d 6 ) δppm: 3.81 (3H, s), 6.99 (1H, d, J = 15.2Hz), 7.09 (2H, m), 8.05 (2H, m), 8.21 (1H, d, J = 15.2Hz).
[0031] MS (ESI, m / z): 207.1 (M + +1, 100%).
[0032] (b) Preparati...
Embodiment 24
[0036] The preparation of embodiment 24-benzyloxybenzoyl ethyl acrylate
[0037] (a) Preparation of 4-benzyloxybenzoic acid
[0038] 10.68 g of benzyl bromide (62.44 mmol) was used instead of methyl iodide, and according to the method of step (a) of Example 1, 14.1 g of off-white powdery solid 4-benzyloxybenzoacrylic acid was obtained, with a yield of 96%. The test results are as follows:
[0039] MS (ESI, m / z): 283.3 (M + +1, 100%).
[0040] (b) Preparation of 4-benzyloxybenzoyl ethyl acrylate
[0041] Using 100mL of ethanol instead of methanol as a solvent, and using the above-prepared 10g of 4-benzyloxybenzoylacrylic acid (35.42mmol) as a raw material, according to the method of step (b) of Example 1, 9.89g of off-white powdery solid 4 was obtained. -Benzyloxybenzoyl ethyl acrylate, yield 90%.
[0042] The test results are as follows:
[0043] 1 H NMR (400MHz, DMSO-d 6 )δppm: 1.32 (3H, m), 4.19 (2H, m), 5.22 (2H, s), 6.58 (1H, d, J=15.2Hz), 7.10 (2H, m), 7.34-7.47 (...
Embodiment 34
[0045] The preparation of embodiment 34-cyclohexyloxybenzoyl ethyl acrylate
[0046] (a) Preparation of 4-cyclohexyloxybenzoic acid
[0047] Using 7.41g of chlorocyclohexane (62.44mmol) instead of methyl iodide, according to the method of step (a) of Example 1, 13.28g of off-white powdery solid 4-cyclohexyloxybenzoacrylic acid was obtained, with a yield of 93%. The test results are as follows:
[0048] MS (ESI, m / z): 275.3 (M + +1, 100%).
[0049] (b) Preparation of 4-cyclohexyloxybenzoyl ethyl acrylate
[0050] Using 100mL of ethanol instead of methanol as a solvent, and using the above-prepared 10g of 4-cyclohexyloxybenzoylacrylic acid (36.46mmol) as a raw material, according to the method of step (b) of Example 1, 9.04g of off-white powdery solids were obtained 4-Cyclohexyloxybenzoyl ethyl acrylate, yield 86%.
[0051] The test results are as follows:
[0052] 1 H NMR (400MHz, DMSO-d 6 )δppm: 1.43-1.95 (10H, m), 3.60 (1H, m), 3.82 (3H, s), 6.56 (1H, d, J=15.2Hz), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com