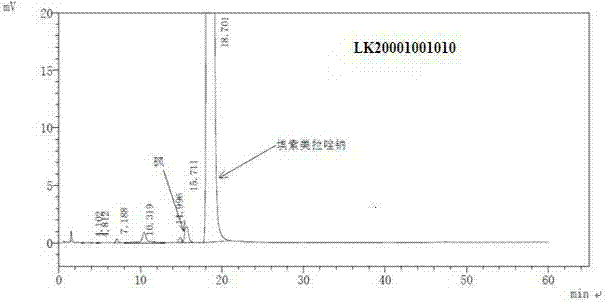
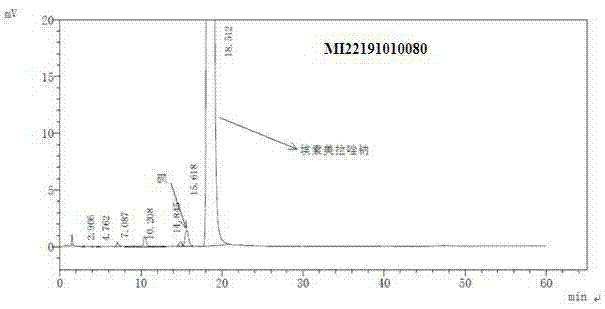
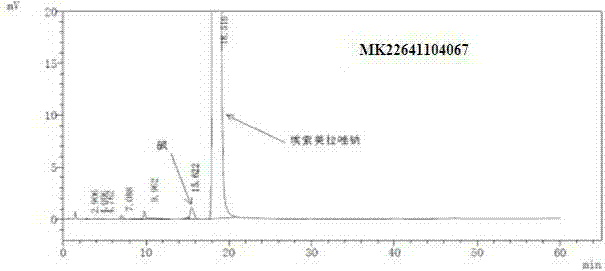
Pharmaceutical composition containing esomeprazole sodium, and preparation method thereof
A technology of esomeprazole sodium and its composition, which is applied in the field of esomeprazole sodium pharmaceutical composition and its preparation, and achieves the effects of high yield, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Put 7.0g (0.212mol) of omemersulfide and 46ml of ethyl acetate into the reaction flask, start stirring, and add in sequence 0.145g of purified water, 4.48g of D-(-)diethyl tartrate, 3.12g of VI isopropyl titanate g. Raise the temperature to 55°C, react for 1 hour, cool down to 20°C, add 1.9ml of triethylamine, then dropwise add 4.38mL of 70% cumene hydroperoxide, and react for 2 hours. After the reaction, separate the organic phase, combine the water layer, add 6mL methyl isobutyl ketone to the water layer, cool in an ice-water bath, add glacial acetic acid under vigorous stirring to adjust the pH to 6.5-7.5, separate the organic layer, and reuse the water layer Extract once with 6 mL of methyl isobutyl ketone, combine the organic layers, and wash once with 6 mL of saturated brine to obtain 25 ml of a methyl isobutyl ketone solution of esomeprazole.
[0102] Add the methyl isobutyl ketone solution of esomeprazole into the reaction flask, start stirring, add methanol so...
Embodiment 2
[0105] 7.0 g (0.212 mol) of omemersulfide was put into the reaction flask, and 2.6 g of crude esomeprazole sodium was prepared according to the method of Example 1.
[0106] Put the crude product of esomeprazole sodium into the reaction kettle, add the mixed solution of methanol and acetonitrile (methanol 1.8mL, acetonitrile 3.6mL), stir at room temperature until completely dissolved. After filtration, 18ml of acetonitrile was added to the filtrate. While stirring, the temperature was maintained at 30°C, and crystallization was carried out for 30 hours. Put the material into the filter for suction filtration, wash the filter cake with acetonitrile after pumping dry, and pump dry again. Obtained 1.76g of esomeprazole sodium refined product (refining yield: 67.6%), which contains 5-(-)-methoxy-2-[[(4-methoxy-3,5-di Methyl-2-pyridyl)methyl]sulfonyl]-1H-benzimidazole sodium 0.07%, except 5-(-)-methoxy-2-[[(4-methoxy-3,5- The maximum amount of single impurity other than dimethyl...
Embodiment 3
[0108]Put 7.0 g (0.212 mol) of omeprazole into the reaction flask, and prepare 2.7 g of crude esomeprazole sodium according to the method of Example 1.
[0109] Put the crude product of esomeprazole sodium into the reaction kettle, add a mixed solution of methanol and acetonitrile (2.2 mL of methanol, 2.2 mL of acetonitrile), and stir at room temperature until completely dissolved. After filtration, 13.2 ml of acetonitrile was added to the filtrate. The temperature was maintained at 0°C under stirring, and crystallization was carried out for 18 hours. Put the material into the filter for suction filtration, wash the filter cake with acetonitrile after pumping dry, and pump dry again. Obtain 1.5g of esomeprazole sodium refined product (refining yield: 55.5%), this refined product contains 5-(-)-methoxy-2-[[(4-methoxy-3,5-di Methyl-2-pyridyl)methyl]sulfonyl]-1H-benzimidazole sodium 0.12%, except 5-(-)-methoxy-2-[[(4-methoxy-3,5- The maximum amount of single impurity other tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com