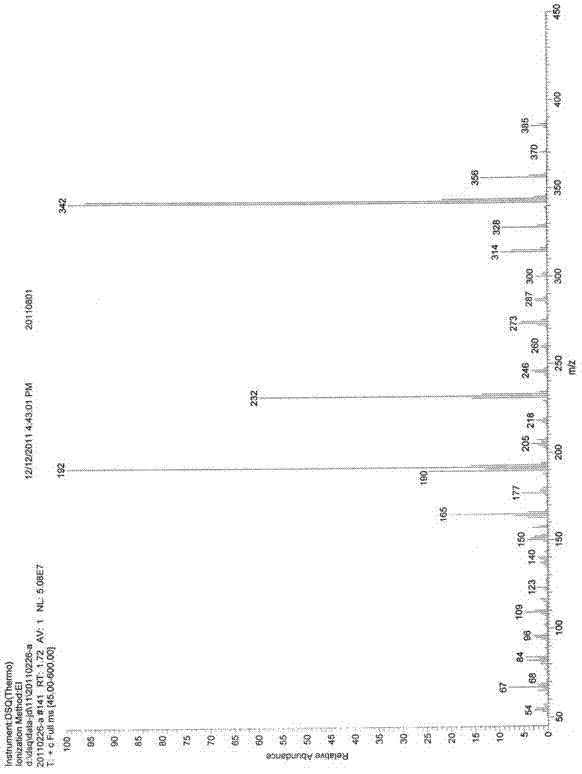
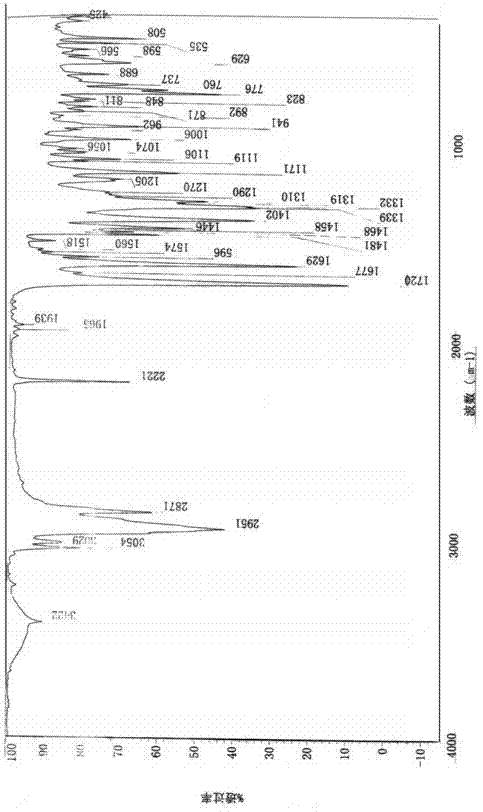
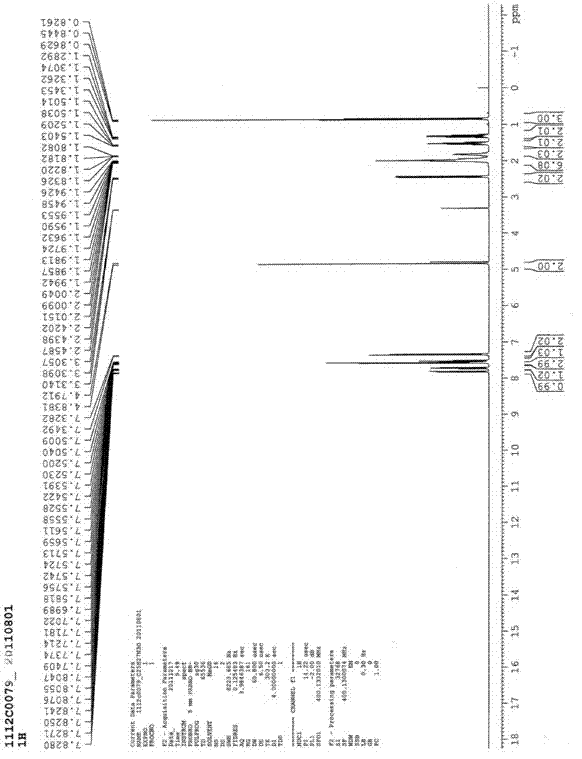
Preparation method of irbesartan
A technology based on irbesartan and cyano, which is applied in the field of preparation of irbesartan, can solve the problems of difficult handling, dangerous use and storage, high price, etc., and achieve the effects of improving quality, easy operation, and reducing the discharge of "three wastes"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment one: the synthesis of erbe hydrocarbon compound
[0049] Add 400g of dichloromethane into a 500ml reaction tank, add 118g (0.433mol) of 2-cyano-4'-bromomethylbiphenyl at room temperature, stir for 10 minutes to dissolve; then add 200g of purified water, 100g ( 0.433mol) 2-butyl-1,3-diazaspiro[4,4]non-1-en-4-one hydrochloride and 5g benzyltriethylammonium chloride, stirred for 10 minutes to make Dissolve; add 400g of NaOH aqueous solution with a mass concentration of 10% dropwise into the reaction tank at 20°C; after the dropwise addition, take a sample for inspection, and the reaction is completed in about 3 to 4 hours.
[0050] Stand still for 30 minutes after the completion of the reaction, separate the dichloromethane layer and wash once with pure water; concentrate the dichloromethane layer under reduced pressure to obtain an oily crude product; add 50 g of acetone to the oily crude product for recrystallization, filter, and dry to obtain Purified produc...
Embodiment 2
[0051] Embodiment two: the synthesis of erbe hydrocarbon compound
[0052] Add 300g of dichloromethane into a 500ml reaction tank, add 125g (0.459mol) of 2-cyano-4'-bromomethylbiphenyl at room temperature, stir for 10 minutes to dissolve; then add 200g of purified water, 100g ( 0.433mol) 2-butyl-1,3-diazaspiro[4,4]non-1-en-4-one hydrochloride and 3g tetrabutylammonium bromide, stirred for 10 minutes to dissolve; At 20°C, add 450 g of KOH aqueous solution with a mass concentration of 5% dropwise into the reaction tank; after the dropwise addition, take a sample for detection, and the reaction is completed in about 3 to 4 hours.
[0053] Stand still for 30 minutes after the completion of the reaction, separate the dichloromethane layer and wash once with pure water; concentrate the dichloromethane layer under reduced pressure to obtain an oily crude product; add 50 g of acetone to the oily crude product for recrystallization, filter, and dry to obtain Purified product 2-butyl-3...
Embodiment 3
[0054] Embodiment three: the synthesis of erbe hydrocarbon compound
[0055] Add 300g of dichloromethane into a 500ml reaction tank, add 285g (1.047mol) of 2-cyano-4'-bromomethylbiphenyl at room temperature, stir for 10 minutes to dissolve; then add 430g of purified water, 210g ( 0.911mol) 2-butyl-1,3-diazaspiro[4,4]non-1-en-4-one hydrochloride and 6g benzyltriethylammonium chloride, stirred for 10 minutes to make Dissolve; add 700g of KOH aqueous solution with a mass concentration of 8% dropwise into the reaction tank at 20°C; after the dropwise addition, take a sample for inspection, and the reaction is completed in about 3 to 4 hours.
[0056] Stand still for 30 minutes after the completion of the reaction, separate the dichloromethane layer and wash once with pure water; concentrate the dichloromethane layer under reduced pressure to obtain an oily crude product; add 105g of acetone to the oily crude product for recrystallization, filter, and dry to obtain Purified produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com