Resource treatment method of CODCr (chemical oxygen demand chromium) measurement waste liquid
A treatment method and recycling technology, applied in the field of waste liquid recycling, can solve environmental hazards and other problems, and achieve the effects of high recovery rate, easy operation, and low recycling cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
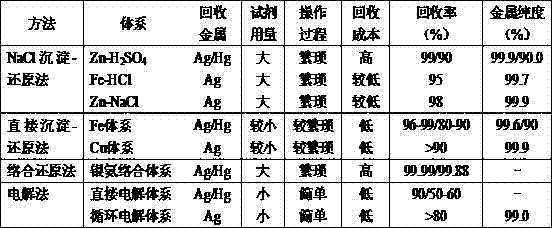
Method used
Image
Examples
Embodiment 1
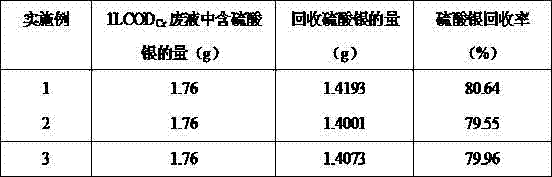
[0049] Take COD Cr Measure waste liquid 1000mL, add 0.9581g sodium chloride, fully react, generate 2.1359g silver chloride, add appropriate amount of concentrated sulfuric acid after filtering, washing and drying, heat to 320°C, react for 20min, filter, wash and dry, the generated Hydrogen chloride gas is absorbed with lye to obtain 1.4193g of product silver sulfate.
Embodiment 2
[0051] Take COD Cr Measure waste liquid 1000mL, add 1.9057g sodium chloride, fully react, generate 2.0641g silver chloride, add appropriate amount of concentrated sulfuric acid after filtering, washing and drying, heat to 320°C, react for 21min, filter, wash and dry, the generated Hydrogen chloride gas is absorbed with lye, obtains product silver sulfate 1.4001g.
Embodiment 3
[0053] Take COD Cr Measure waste liquid 1000mL, add 2.8568g sodium chloride, fully react, generate 2.0789g silver chloride, filter, wash, dry, add appropriate amount of concentrated sulfuric acid, heat to 320°C, react for 22min, filter, wash, dry, the generated Hydrogen chloride gas is absorbed with lye to obtain product silver sulfate 1.4073g.
[0054] Table 2 Test results
[0055]
[0056] As can be seen from Table 2, the method has a high recovery rate, which can reach more than 80.6%; the recovery rate is stable, all around 80%; and no secondary pollution will be produced.
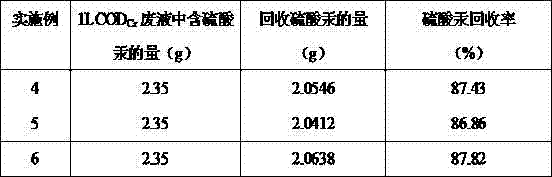
[0057] What adopted in the embodiment is the waste liquid produced by the national standard GB11914-89 method, containing about 2.35g of mercury sulfate in every liter of waste liquid. The implementation results are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com