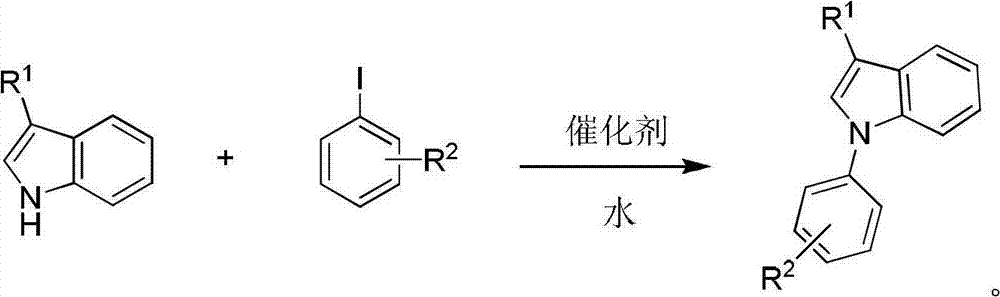
Catalyst for aqueous-phase preparation of indole nitrogen arylide and preparation method of indole nitrogen arylide
A technology for the preparation of indole nitrogen aromatics and water phases, applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem of no catalyst, no water phase synthesis method and other problems, to achieve the effect of simple composition, easy control of reaction conditions and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Cuprous iodide (8 mg, 0.04 mmol), 2,2'-bipyridine (12 mg, 0.08 mmol), betaine (94 mg, 0.8 mmol), tripotassium phosphate (170 mg, 0.8 mol), indole (47 mg, 0.4 mmol), and p-methoxyiodobenzene (187 mg, 0.8 mmol) were placed in a reaction flask, 2 ml of water were added, heated to 90 degrees Celsius, and reacted for 10 hours. Chloroform was added to the reaction system, the product was extracted with chloroform, concentrated and separated by silica gel chromatography to obtain 72 mg of indole nitrogen-p-methoxybenzene with a yield of 81%.
[0033] NMR data of indole nitrogen p-methoxybenzene: 1 H NMR (500MHz, CDCl 3 )δ7.67(d, J=8.0Hz, 1H), 7.45(d, J=8.0Hz, 1H), 7.40(d, J=9.0Hz, 2H), 7.27(d, J=3.5, 1H), 7.20(t, J=8.0, 1H), 7.14(t, J=8.0, 1H), 7.02(d, J=9.0, 2H), 6.64(d, J=3.5, 1H), 3.87(s, 3H) .
Embodiment 2
[0035] Cuprous iodide (8 mg, 0.04 mmol), 2,2'-bipyridine (12 mg, 0.08 mmol), betaine (94 mg, 0.8 mmol), tripotassium phosphate (170 mg, 0.8 mol), indole (47 mg, 0.4 mmol), and p-nitroiodobenzene (199 mg, 0.8 mmol) were placed in a reaction flask, 2 ml of water were added, heated to 90 degrees Celsius, and reacted for 10 hours. Chloroform was added to the reaction system, the product was extracted with chloroform, concentrated and separated by silica gel chromatography to obtain 90 mg of indole nitrogen-p-nitrobenzene with a yield of 95%.
[0036] NMR data of indole nitrogen p-nitrobenzene: 1 H NMR (500MHz, CDCl 3 )δ8.42-8.37(m, 2H), 7.72-7.64(m, 4H), 7.37(d, J=3.5Hz, 1H), 7.32-7.21(m, 2H), 6.76(d, J=3.5Hz , 1H).
Embodiment 3
[0038] Cuprous iodide (8 mg, 0.04 mmol), 2,2'-bipyridine (12 mg, 0.08 mmol), betaine (94 mg, 0.8 mmol), tripotassium phosphate (170 mg, 0.8 mol), indole (47 mg, 0.4 mmol), and p-chloroiodobenzene (191 mg, 0.8 mmol) were placed in a reaction flask, 2 ml of water were added, heated to 90 degrees Celsius, and reacted for 10 hours. Chloroform was added to the reaction system, the product was extracted with chloroform, concentrated and separated by silica gel chromatography to obtain 74 mg of indole nitrogen p-chlorobenzene with a yield of 82%.
[0039] NMR data of indole nitrogen p-chlorobenzene: 1 H NMR (500MHz, CDCl 3 )δ7.68(d, J=8.0Hz, 1H), 7.52-7.41(m, 5H), 7.28(d, J=3.0Hz, 1H), 7.25-7.15(m, 2H), 6.68(d, J =3.0Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com