A novel azole antifungal compound and its preparation method and application
A technology of antifungal drugs and compounds, applied in antifungal agents, organic chemistry, etc., to achieve high yield, simple preparation method and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
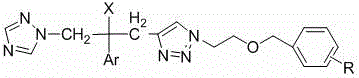
[0035] R group and proton nuclear magnetic spectrum data of embodiment 1 part compound of the present invention
[0036] See Table 1 for the R groups and H NMR spectrum data of some compounds of the present invention.
[0037] Table 1 R group and proton nuclear magnetic spectrum data table of some compounds of the present invention
[0038] serial number
[0039] Note: The number in the R group in the table represents the position of substitution on the benzene ring.
Embodiment 2
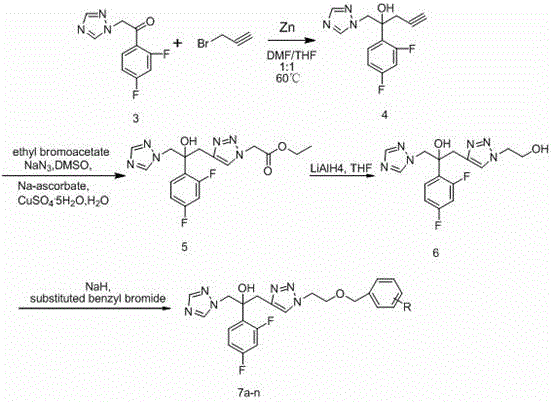
[0040] The preparation of the intermediate of embodiment 2 compounds of the present invention
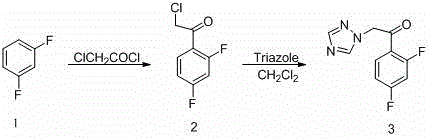
[0041] 1. Preparation of 2-chloro-2′,4′-difluoroacetophenone
[0042] Put 200g (1.494mol) of anhydrous aluminum trichloride and 150g (1.30mol) of m-difluorobenzene into a 1000mL three-necked bottle, stir at room temperature, and slowly add 150g (1.30mol) of chloroacetyl chloride dropwise. Continue to stir at room temperature for 30 minutes, slowly raise the temperature to 45°C, continue to stir at this temperature for 4.5 hours, pour the reaction solution into ice water as usual, precipitate a solid, and filter; the filtrate is extracted twice with 800 mL of dichloromethane, The dichloromethane extracts were combined, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered to obtain a solid, which was combined twice and recrystallized with ethanol to obtain 2-chloro 2′,4′-difluorophenethyl Ketone 215g, yield 88.2%, melting point...
Embodiment 3
[0051] Embodiment 3 Preparation of some compounds of the present invention
[0052] 1. Preparation of 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[1-(2-fluorobenzyloxy)ethyl- 1H-1,2,3triazol-4-yl)]-2-ol (7a)
[0053] Take the compound 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[1-hydroxyethyl-1H- 1,2,3 Triazol-4-yl)]-2-ol 350 mg (1.0 mmol) in 20 mL dry CH 2 Cl 2 26mg (1.1mmol) of NaH was added slowly under the condition of ice bath, and after stirring for half an hour in the ice bath, 0.13mL (1.2mmol) of o-fluorobenzyl bromide was added, the reaction conditions remained unchanged, and the reaction was completed in about 2 hours. , add 20mL CH 2 Cl 2 Dilute, add a small amount of methanol slowly under ice bath to remove the remaining NaH, then wash with water and saturated brine, evaporate the solvent to dryness, and purify by column chromatography (developing solvent: ethyl acetate:petroleum ether=1:1) to obtain white Solid target compound 1-(1H-1,2,4-tria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com