Novel azole antifungal compound and preparation method and application thereof
A compound, nitrogen azole technology, applied in the field of novel nitrogen azole antifungal compound and its preparation, achieving high yield, strong antifungal activity and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
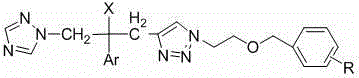
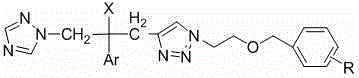
[0035] Example 1 R group and proton nuclear magnetic spectrum data of some compounds of the present invention
[0036] See Table 1 for the R groups and H NMR spectrum data of some compounds of the present invention.
[0037] Table 1 R group and proton nuclear magnetic spectrum data table of some compounds of the present invention
[0038] serial number R 1 HNMR (300MHz, CDCl 3 ) 7a 2-F 8.27 (1H, s, 1,2,4-triazole-H), 7.75 (1H, s, 1,2,4-triazole-H), 7.58 (1H, s, 1,2,3-triazole-H) , 7.27-6.77 (7H, m, Ar-H), 5.98 (1H, s, OH), 4.69-4.48 (2H, dd, J =14.4Hz, 1,2,4-triazole-CH 2 ), 4.44 (2H, s, CH 2 * CH 2 O), 4.40 (2H, s, ArCH 2 O), 3.74-3.71 (2H, t, CH 2 CH 2 * O), 3.35-3.10 (2H, dd, J =15.0Hz, 1,2,3-triazole-CH 2 * C(OH)) . 7b 3-F 8.27 (1H, s, 1,2,4-triazole-H), 7.75 (1H, s, 1,2,4-triazole-H), 7.60 (1H, s, 1,2,3-triazole-H) , 7.35-6.77 (7H, m, Ar-H), 5.98 (1H, s, OH), 4.69-4.49 (2H, dd, J =14.1Hz, 1,2,4-triazole-CH 2 ), 4.4...
Embodiment 2
[0040] Embodiment 2 The preparation of the intermediate of compound of the present invention
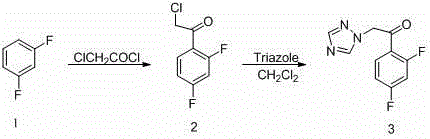
[0041] 1. Preparation of 2-chloro-2′, 4′-difluoroacetophenone
[0042] Put 200 g (1.494 mol) of anhydrous aluminum trichloride and 150 g (1.30 mol) of m-difluorobenzene into a 1000 mL three-neck flask, stir at room temperature, and slowly add 150 g (1.30 mol) of chloroacetyl chloride dropwise, After the dropwise addition, continue to stir at room temperature for 30 minutes, slowly raise the temperature to 45°C, and continue to stir at this temperature for 4.5 hours. Pour the reaction solution into ice water as usual, precipitate solids, and filter; the filtrate is dichloromethane 800 mL Extract twice, combine the dichloromethane extracts, wash with water until neutral, dry over anhydrous sodium sulfate, filter, recover the solvent to obtain a solid, combine the solid obtained twice and recrystallize with ethanol to obtain 2-chloro 2',4' - Difluoroacetophenone 215 g, yield 88...
Embodiment 3
[0051] Embodiment 3 Preparation of some compounds of the present invention
[0052] 1, preparation 1-(1 H -1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[1-(2-fluorobenzyloxy)ethyl-1 H -1,2,3triazol-4-yl)]-2-ol (7a)
[0053] Get the compound 1-(1 that embodiment 2 prepares H -1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[1-hydroxyethyl-1 H -1,2,3triazol-4-yl)]-2-ol 350 mg (1.0 mmol) in 20 mL dry CH 2 Cl 2 Add 26 mg (1.1 mmol) of NaH slowly under ice bath conditions, keep stirring in the ice bath for half an hour, then add 0.13 mL (1.2 mmol) of o-fluorobenzyl bromide, the reaction conditions remain unchanged, and the reaction ends in about 2 hours. At the end, add 20 mL CH 2 Cl 2 Dilute, add a small amount of methanol slowly under ice bath to remove the remaining NaH, then wash with water and saturated brine, evaporate the solvent to dryness, and purify by column chromatography (developing solvent: ethyl acetate:petroleum ether=1:1) to obtain white Solid target compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com