Cdc42 inhibitor and application thereof
A technology of cdc42 and inhibitors, applied in the field of compounds, can solve the problem of no selective inhibitors of Cdc42 and achieve the effect of reducing accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Virtual Screening of Cdc42 Inhibitors
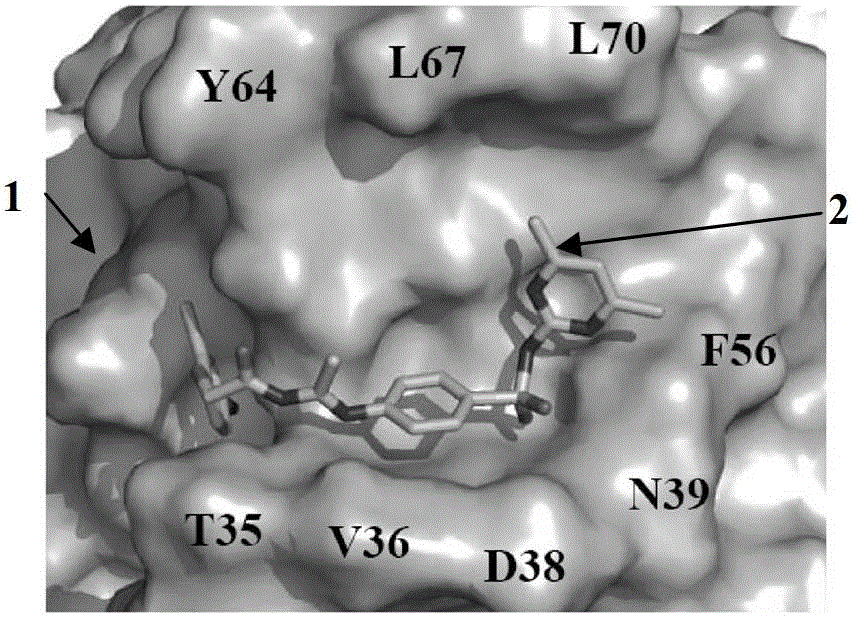
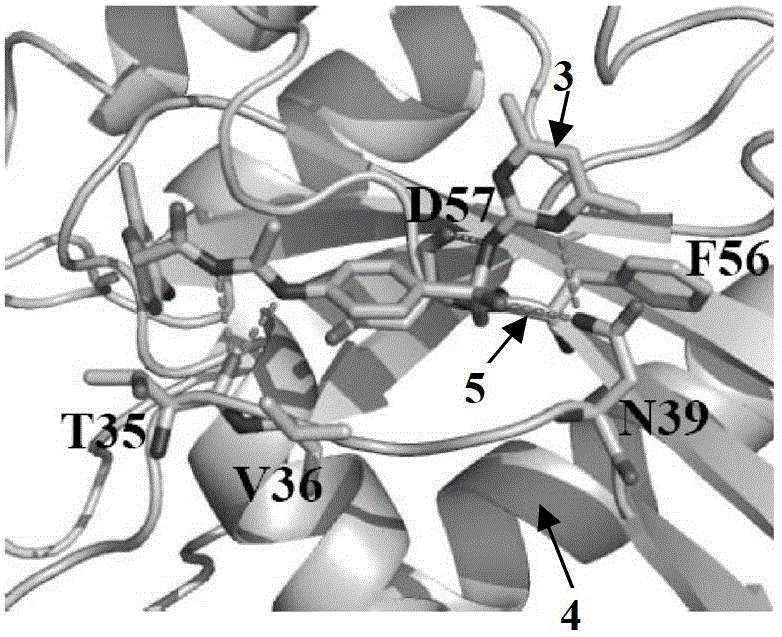
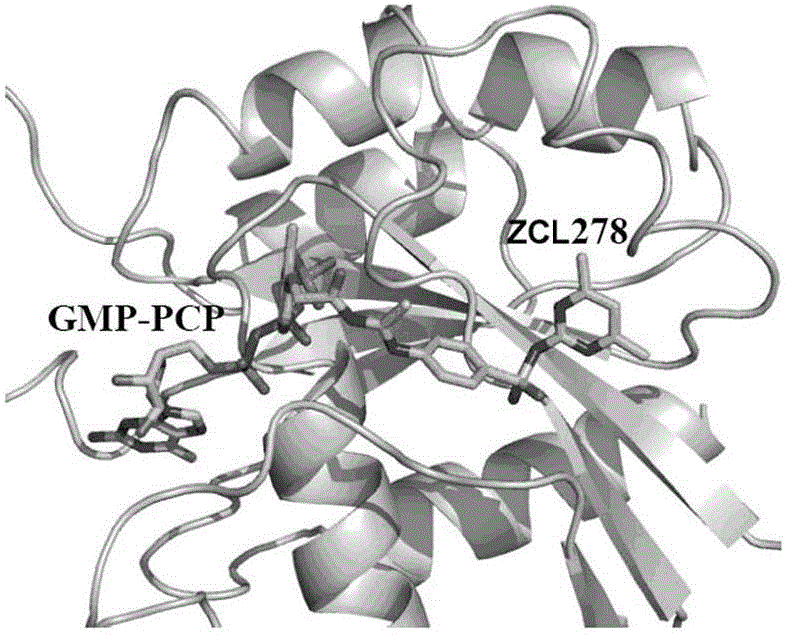
[0030]Analysis of the three-dimensional structure of the Cdc42-ITSN complex revealed a major binding domain between the two molecules, the hydrogen bond between the Gln1380 residue and the Arg1384 residue in the ITSN molecule and between Asn39 and Phe37 on Cdc42, and the hydrogen bond in the ITSN molecule Two hydrophobic clusters between residues Leu1376, Met1379 and Thr1383 and between Phe56, Tyr64, Leu67 and Leu70 on Cdc42. In order to screen Cdc42 inhibitors, it is presumed that the binding pocket on the Cdc42 molecule is a structure formed by amino acid residues within a radius distance of 7A from the ITSN protein surface that binds to Cdc42. This contains 16 amino acid residues such as Thr35, Val36, Asn39, Phe56 and Asp57 in the Cdc42 protein molecule, such as Figure 1A-Figure 1C shown.
[0031] The Glide program was used to screen the small molecular compounds that could disrupt the connection between Cdc42 and ...
Embodiment 2
[0033] Embodiment 2: the synthesis of compound ZCL278:
[0034] ZCL278 can be synthesized by the following synthetic method, the following synthetic method is only for illustration, not limitation of the present invention, those skilled in the art can understand and imagine that other synthetic methods can be used to synthesize ZCL278, which also belongs to the protection scope of the present invention .
[0035] Reagents and conditions: (a) K 2 CO 3 , DMF (N, N-dimethylformamide), 70°C; (b) NaOH, dioxane (dioxane) / H 2 O; (c) SOCl 2 (thionyl chloride), DMF, reflux (reflux); (d) NaSCN (sodium thiocyanate), acetone (acetone), 0°C-rt; (e) 4-amino-N-(4,6-dimethylpyrimidin -2-yl)benzene-sulfonamide (4-amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide), 0°C-r.t.
[0036] The reaction formula is as follows:
[0037]
[0038] Compound 1 (4-bromo-2-chlorophenol) and ethyl 2-bromoacetate in K 2 CO 3 Nucleophilic substitution reaction in the presence can afford compound 2...
Embodiment 3
[0046] Embodiment 3: ZCL278 active characteristic
[0047] 1. ZCL278 inhibits Cdc42-mediated microspine formation
[0048] The effects of 30 candidate compounds on Cdc42-mediated microspike / filopodia formation were tested on serum-free mouse fibroblast Swiss 3T3 cells. Microspines / filopodia composed of fibroblast actin characterize Cdc42 activity. Such as Figure 2A As shown, a few micro spines (indicated by small arrows) and characteristic stress fibers mediated by RhoA (indicated by asterisks) can be seen at the edge of the cells in the control group. After the cells were briefly stimulated with 1 unit / mL of Cdc42 agonist (cytoskeleton company), the number of micro spines increased and the number of stress fibers decreased obviously. Adding the above agonists for 2 minutes, and then treating the cells with 50uM ZCL278 for 1 hour, ZCL278 significantly inhibited the formation of microthorns compared to the agonist alone. This result shows that the compound can be used as a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com