Preparation method of black-bone chicken oligopeptide and separation and identification method of active peptide fragment
An identification method and oligopeptide technology are applied in the field of biomedicine to achieve the effects of high yield, improved nutritional value and high degree of hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]1.83kg white striped black-bone chicken was made into meat puree with a chopper and a meat grinder, transferred to a hydrolysis pot, added 5L of deionized water, stirred evenly, heated and boiled for 0.5h, and cooled to room temperature. After the liquid material was treated with a colloid mill, it was heated to 90°C, and allowed to stand and cooled to room temperature. Centrifuge the liquid material at a speed of 3000r / min for 15 minutes, let it stand for 5 minutes, discard the upper layer of the liquid material, and keep the lower layer of liquid material. Return the liquid material to the hydrolysis kettle, heat to 50°C, adjust the liquid material to pH 7.5 with NaOH solution, add Protamex enzyme with a solid content of 3.0% in the liquid material, and enzymolyze the protein until the degree of protein hydrolysis of the liquid material reaches about 70%. The liquid material is passed through a high temperature instantaneous sterilization (UHT) system to kill enzymes. ...
Embodiment 2
[0067] 1902.3kg white striped black-bone chicken was made into meat puree with a chopping machine and a meat grinder, transferred to a hydrolysis tank, added 6000L of deionized water, stirred evenly, heated and boiled for 0.5h, and cooled to room temperature. After refining the liquid material with a colloid mill, heat it to 90°C, and let it stand to cool down to room temperature. Process the liquid material with a disc centrifuge to remove grease and retain the protein liquid material. Return the liquid material to the hydrolysis tank, heat to 55°C, adjust the liquid material to pH 8.5 with NaOH solution, add Protamex enzyme with a solid content of 5.0% in the liquid material, and perform enzymatic hydrolysis until the protein hydrolysis degree of the liquid material reaches about 70%. The liquid material is passed through a high temperature instantaneous sterilization (UHT) system to kill enzymes. Add ionized water to the liquid material to a solid content of 5%, and after ...
Embodiment 3
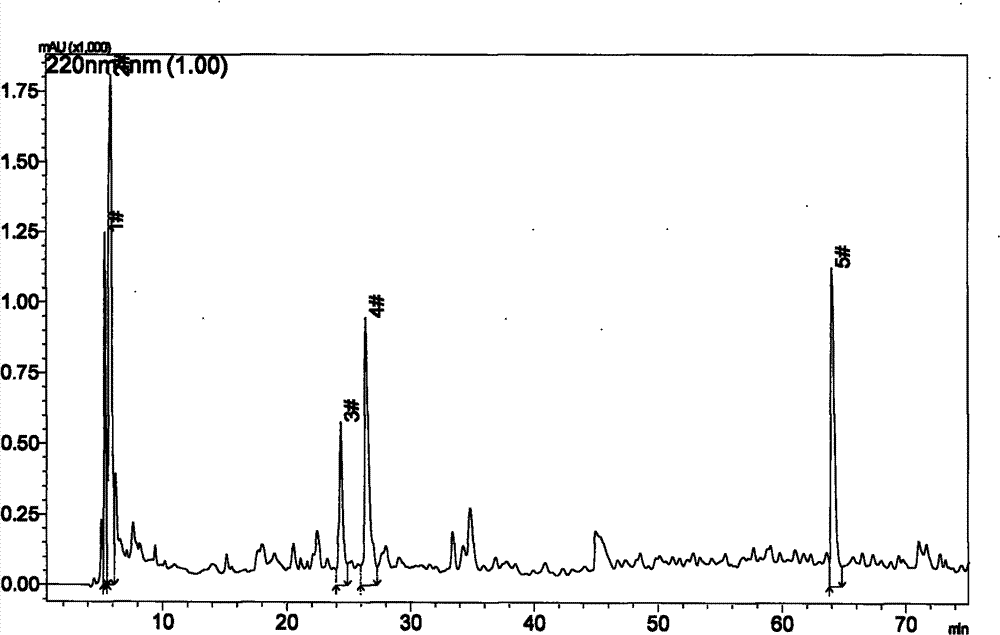
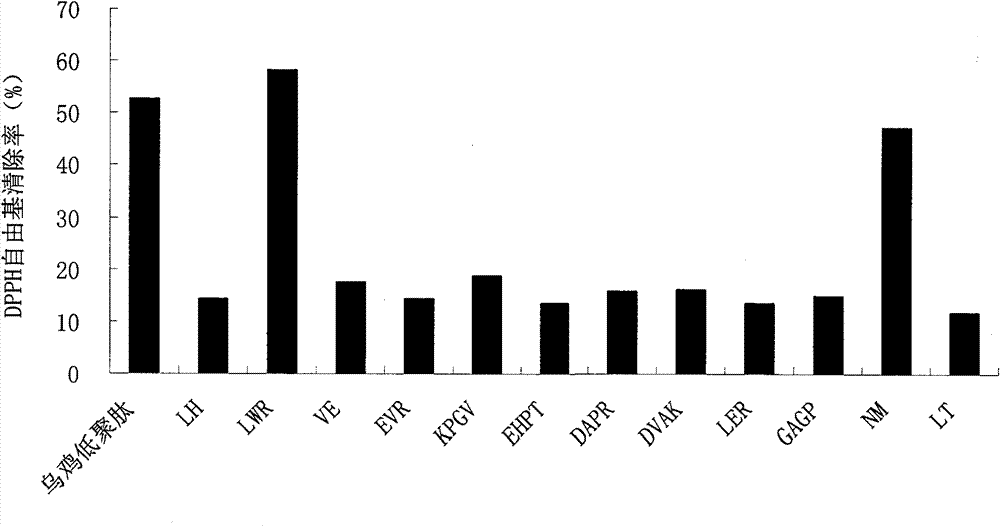
[0069] Use RP-HPLC to separate the components of black-bone chicken oligopeptides. The operation steps are: (1) Sample preparation: Weigh 1g of black-bone chicken oligopeptide dry powder, dilute it to a 100mL volumetric flask with mobile phase A, and oscillate ultrasonically for 10 minutes. The sample was fully dissolved and mixed to prepare a 0.1 mg / mL black-bone chicken oligopeptide solution. The sample solution was filtered with a polytetrafluoroethylene filter membrane with a pore size of 0.2 μm. (2) Sample loading: the sample loading volume is 50 μL. (3) Gradient elution: chromatographic column: XBridge BEH130 C18 column (4.6×250mm); mobile phase A: V (water): V (trifluoroacetic acid) = 100: 0.1; mobile phase B: V (acetonitrile): V (Water): V (trifluoroacetic acid) = 80: 20: 0.1; detection wavelength: UV220nm; flow rate: 0.6mL / min; column temperature: 32°C; gradient elution program: 0-20min, mobile phase B: 0% -5%; 20-30min, mobile phase B: 5%-5%; 30-80min, mobile phase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ash content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com