Chinese medicament for treating atrophic gastritis and preparation method thereof
A technology for atrophic gastritis and drugs, applied in the field of drugs for the treatment of atrophic gastritis, can solve the same problems and achieve the effects of easy acceptance, simple and scientific process, and comprehensive indications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
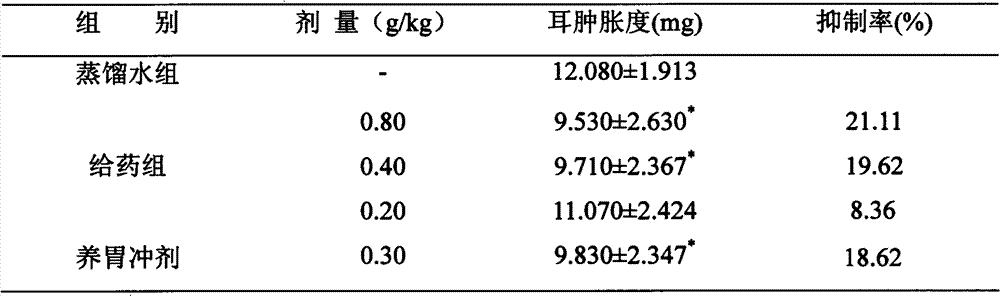
[0022] Animal experiment example 1: Effect of p-xylene on mouse ear swelling
[0023] Observe the effect of the capsule of the present invention on mouse ear swelling caused by xylene. Test drug powder, batch number: 100101; xylene, domestic analytical grade. Yangwei Granules was selected as the positive control drug, batch number: 090403, from Zhengda Qingchunbao Pharmaceutical Co., Ltd. The experimental animals were Kunming mice, provided by the Experimental Animal Center of Fourth Military Medical University.
[0024] Take 50 mice and divide them into 5 groups randomly, 10 mice in each group, with a body weight of 20±2.5g, give the corresponding dose of medicine once a day, for 6 consecutive days, and drop 0.05ml of xylene in the right ear of each mouse after 1 hour of the last administration. Inflammation, the left ear was used as a control, and the left ear was killed after 15 minutes of inflammation. Both ears were cut off, and round ear pieces were respectively punche...
experiment example 2
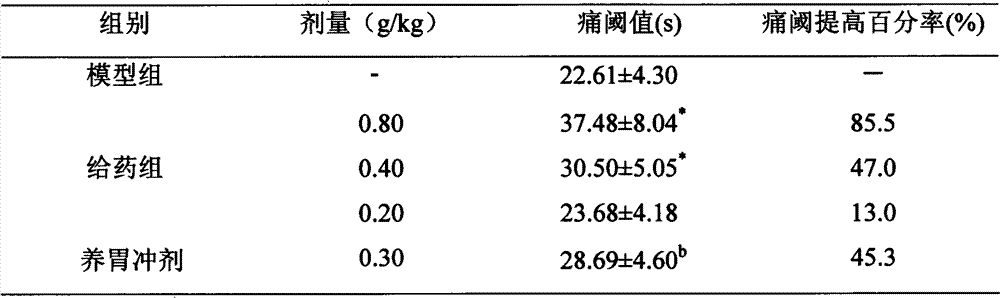
[0028] Animal Experiment Example 2: Effects on Pain in Mice
[0029] (1) Hot plate method: 50 mice were randomly divided into 5 groups, 10 in each group, and the dosage was the same as above. One hour after the last administration, the mice were put into a hot beaker (constant temperature water bath 55°C ± 1°C); and timed immediately, and the time (s) required for the mice to lick their hind feet from being put into the beaker was recorded as the Rat pain threshold, and calculate the pain threshold increase rate, the calculation formula is: [(average pain threshold after medication-average pain threshold of unmedicated group) / average pain threshold of unmedicated group]×100%. The results showed that the test drug 0.80g / kg and 0.40g / kg had significant analgesic effect (P<0.01).
[0030] The impact of table 2 on mouse pain (n=10; )
[0031]
[0032] Note: * P<0.01 compared with model group.
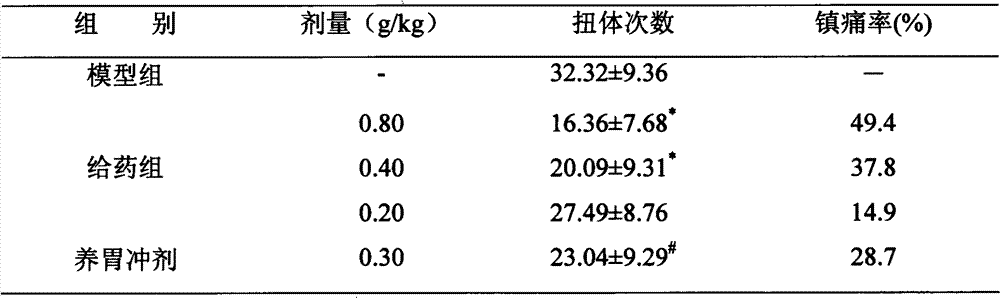
[0033] (2) Writhing method: each mouse was given ip 0.5% acetic acid solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com