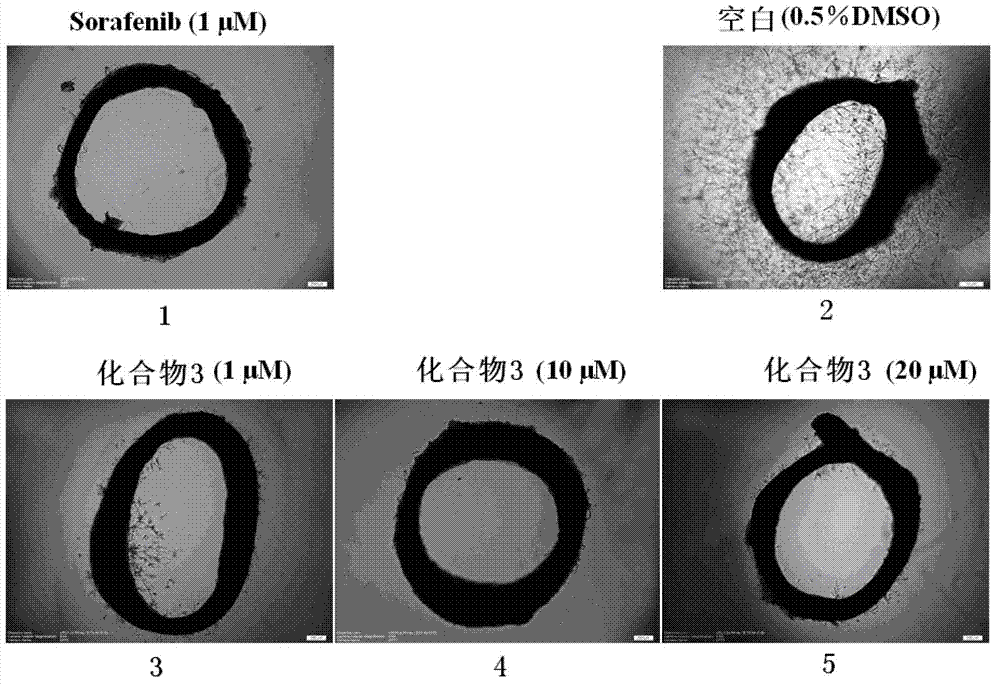
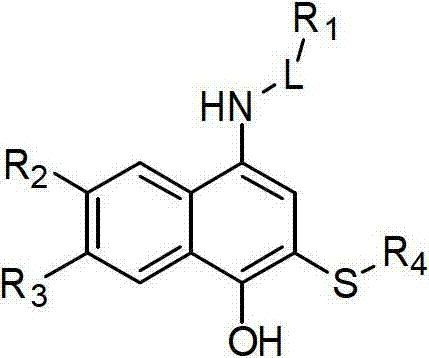
2-sulfo-4-amino-1-naphthol derivative and preparation method and application thereof
A technology of naphthol derivatives and derivatives, applied in drug combinations, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., can solve the problems of tumors' low dependence on angiogenesis, drug resistance, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 Synthesis of N-(3-((1H-1,2,4-triazole-5-)thio)-4-hydroxynaphthalene-1-yl)naphthalene-2-carboxamide (compound 1) :
[0106]
[0107] Compound 1
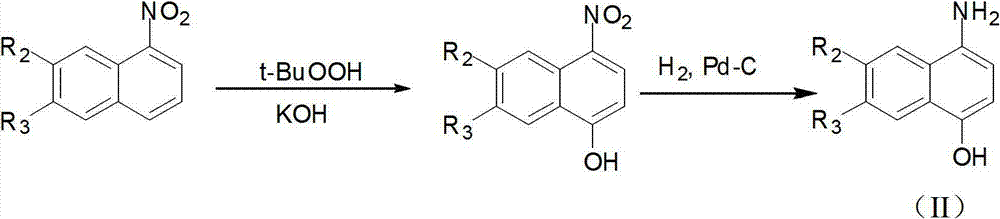
[0108] 1) Synthesis of 4-amino-1-naphthol:
[0109]
[0110] Dissolve 1-nitronaphthalene (0.86g, 5mmol) in 13ml DMSO and cool down to -5~5°C, add 5ml of KOH (1.2g, 20mmol) aqueous solution, stir for 5min, then add t-butanol peroxide dropwise - BuOOH (1.8 g, 10 mmol) in DMSO 2 ml. After stirring at room temperature for about 2 hours, the reaction was complete. The solution was poured into 50ml of 1M hydrochloric acid solution, and a light yellow solid precipitated. After filtering, the filter cake was dried and weighed to obtain 1.38g of crude product. After recrystallization, 1.20 g of pure product was obtained, which was light yellow and had a melting point of 166-168°C. The resulting 4-nitro-1-naphthol (1.89 g 10 mmol) was dissolved in 20 ml CH 3 In OH, hydrogen gas was introduced under 10 atmospheres, an...
Embodiment 2
[0121] Example 2 N-(3-((1H-1,2,4-triazole-5-)thio)-4-hydroxynaphthalene-1-yl)-4-bromo-2-fluorobenzenesulfonamide ( Compound 11) Synthesis:
[0122]
[0123] Compound 11
[0124] 1) Synthesis of 4-amino-1-naphthol
[0125]
[0126] Dissolve 1-nitronaphthalene (0.86g, 5mmol) in 13ml DMSO and cool down to -5~5°C, add KOH (1.2g, 20mmol) in 5ml aqueous solution, stir for 5min, then add tert-butanol peroxide dropwise - BuOOH (1.8 g, 10 mmol) in 2 ml DMSO. Stir at room temperature for about 2 hours. After the reaction is complete, pour the solution into 50ml of 1mol / L hydrochloric acid solution, a light yellow solid precipitates, filter, and weigh the filter cake to obtain 1.38g of crude product after drying. After recrystallization, 1.20 g of pure product was obtained, which was light yellow and had a melting point of 166-168°C.
[0127] The obtained 4-nitro-1-naphthol (189g, 10mmol) was dissolved in 20ml CH 3 In OH, stirred and reacted for 10 hours under 10 atmospheric p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com