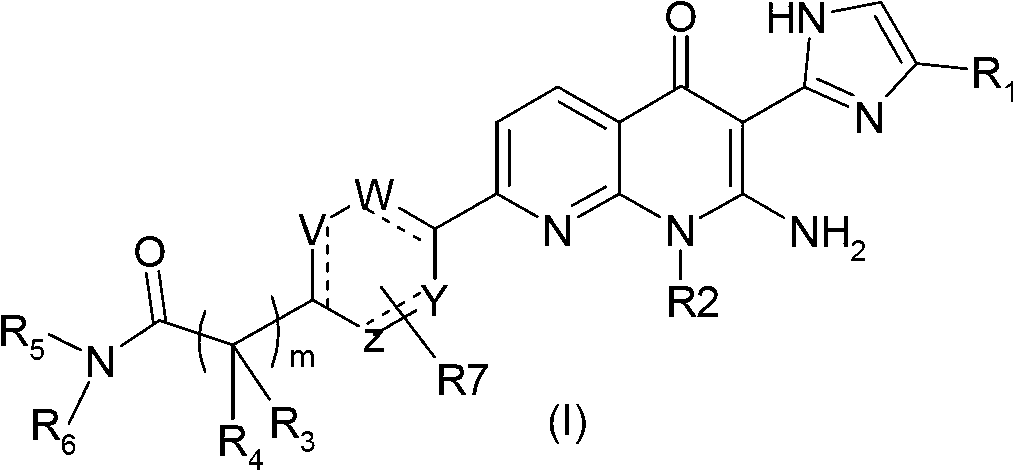
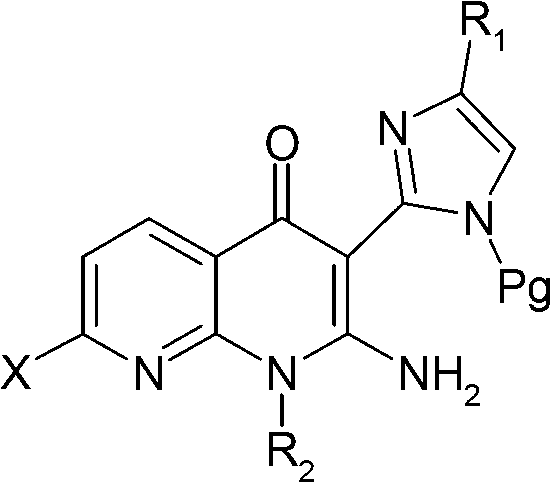
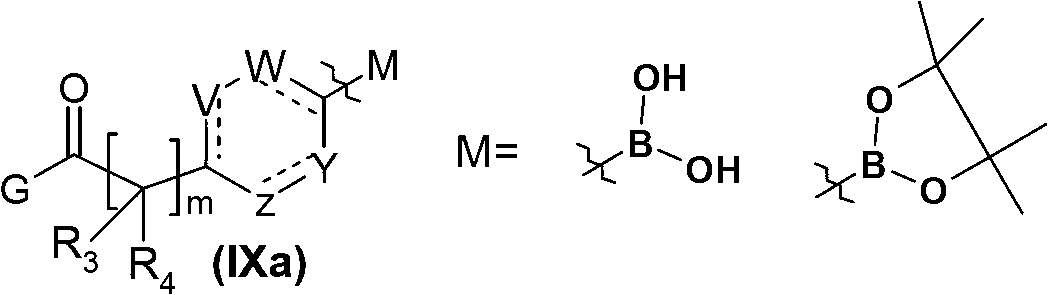
Pyridine-pyridinone derivatives, preparation and therapeutic use thereof
A compound and alkyl technology, applied in the field of pyridopyridone derivatives, can solve the problems such as chemotherapy can not achieve cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0290] Example 1: 2-{6-[7-Amino-8-ethyl-6-(1H-imidazol-2-yl)-5-oxo-5,8-dihydro-[1,8]naphthyridine-2 -yl]-pyridin-3-yl}-N-(pyridin-2-yl)-acetamide (compound 2)
[0291] 1.1 / 6-Chloro-2-(ethylamino)pyridine-3-carboxylic acid
[0292] A solution of 18.0 g (84.4 mmol) of 2,6-dichloropyridine-3-carboxylic acid in 180 ml (3.45 mol) of 70% aqueous ethylamine was heated at 50° C. for 10 hours. The excess amine was then evaporated under reduced pressure, followed by the addition of 10% aqueous acetic acid until the product precipitated. The beige solid was filtered, rinsed with cold water and dried. 10.5 g of the desired product are obtained. Yield = 62%. Melting point: 158-160°C. M H + : 201.1 (Tr: 7.7 minutes, condition 1).
[0293] 1.2 / 6-Chloro-2-(ethylamino)pyridine-3-carbonyl fluoride
[0294] 4.2 ml (52.3 mmol) of pyridine and 8.4 ml (99.6 mmol) of cyanuric fluoride were added successively to a suspension of 10.5 g (52.3 mmol) of the compound obtained in step 1.1 i...
Embodiment 2
[0307] Example 2 : 2-{4-[7-amino-8-ethyl-6-(1H-imidazol-2-yl)-5-oxo-5,8-dihydro-[1,8]phthalazine- 2-yl]-phenyl}-N-(4-cyclopropyl-morpholin-3-ylmethyl)-acetamide (compound 3)
[0308] 2.1 / Ethyl [4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]acetate
[0309] A mixture of 9.5 g (33 mmol) (4-iodo-phenyl)-ethyl acetate and 9.2 g (36 mmol) dipinacol diboronate in anhydrous dimethylsulfoxide (65 ml) was degassed with argon After 15 minutes, 28 g (98 mmol) of potassium acetate and 1.34 g (1.6 mmol) of (phosphinoferrocene)palladium dichloride were added and the reaction mixture was heated at 55° C. under argon for 1.5 hours. The reaction mixture was diluted with 220ml ethyl acetate, then the organic phase was washed three times with water (200ml), then washed with Na 2 SO 4 Dry and concentrate under reduced pressure. 11 g of compound was obtained as a brown oil and used as such in the next step. MH + : 291.2 (Tr: 9.4 minutes, condition 1).
[0310] 2.2 / (4-{7-amin...
Embodiment 3
[0321] Example 3 : 2-{4-[7-amino-8-ethyl-6-(1H-imidazol-2-yl)-5-oxo-5,8-dihydro-[1,8]phthalazine- 2-yl]-phenyl}-N-(pyridin-4-ylmethyl)-propionamide (compound 47)
[0322] 3.1 / 2-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoic acid ethyl ester
[0323] The same operation as described in step 2.1 of Example 2, from a solution of 0.6g (2mmol) ethyl 2-(4-iodophenyl) propionate in anhydrous DMSO (4ml), 0.57g (2.2mmol) diboronic acid Start with dipinacol ester, 1.7 g (6 mmol) potassium acetate and 83 mg (0.1 mmol) (phosphinoferrocene)palladium dichloride. Obtained 0.9 g of compound as a brown oil and used as such in the next step. MH + : 305 (Tr: 9.9 minutes, condition 1).
[0324] 3.2 / 2-{4-[7-Amino-8-ethyl-5-oxo-6-(1-{[2-(trimethylsilyl)ethoxy]methyl Base}-1H-imidazol-2-yl)-5,8-dihydro-1,8-naphthyridine-2-yl]phenyl}propionic acid
[0325] The same operation as described in Example 2 step 2.2, from 0.57g (1.4mmol) of the compound obtained in step 3.1, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com