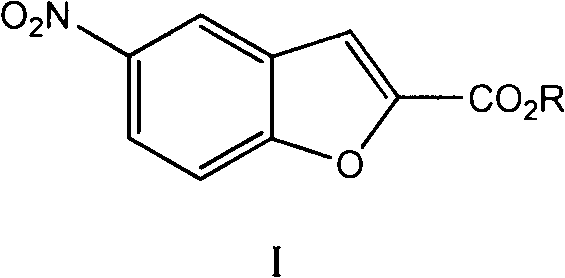
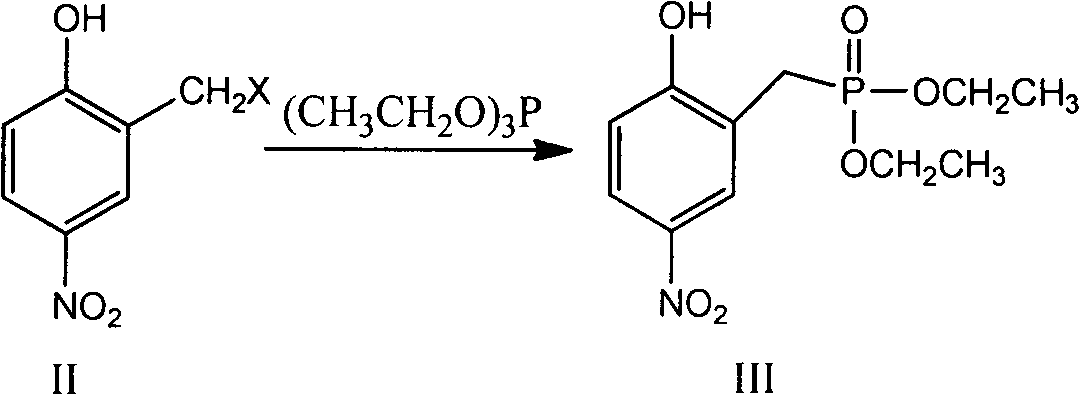
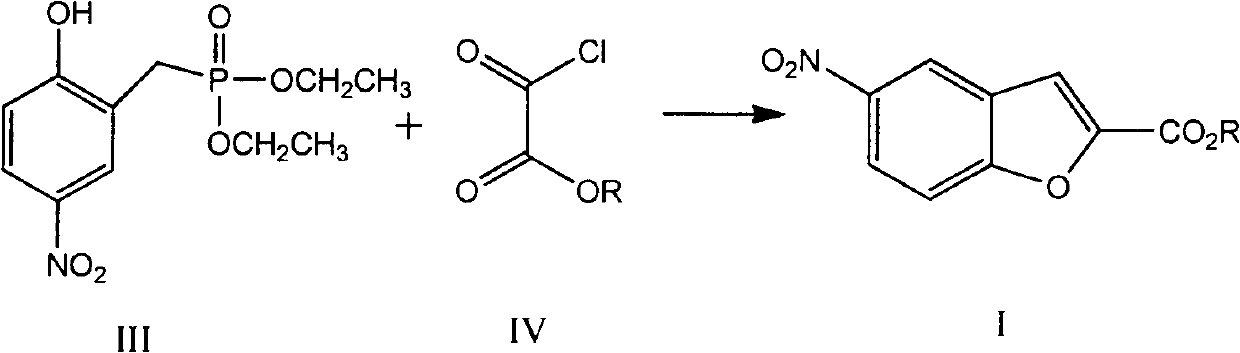
Preparation method of vilazodone intermediate
A technology for vilazodone and intermediates, which is applied in the field of preparation of vilazodone intermediates, can solve the problems of unfavorable large-scale industrial production, difficulties in separation and purification, and low synthesis yield, and achieve the advantages of industrial production and raw materials. Inexpensive and easy to obtain, high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Compound III (Compound IIa is the compound in which X is Cl in Compound II)
[0033]
[0034] Add 18.7g (0.10mol) of 2-hydroxy-5-nitrobenzyl chloride and 18.3g (0.11mol) of triethyl phosphite into the reaction flask, stir and raise the temperature to 100°C, keep it warm for 3.5 hours, and cool it down for use.
Embodiment 2
[0036] Preparation of compound I (compound IVa is the compound in which R is ethyl in compound IV)
[0037]
[0038] Dissolve the compound III obtained in Example 1 in 175ml of anhydrous DMF, control the temperature in an ice bath at 0-5°C, add 27.2g (0.4mol) of sodium ethoxide, and drop 15.0g (0.11mol) of oxalyl chloride at 5°C After the addition of monoethyl ester was completed and reacted at room temperature for 5 hours, 200ml of ice water was added, extracted with ethyl acetate, the organic phase was washed twice with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was recrystallized with methanol to obtain White solid 14.5g, melting point: 140-141°C, total yield 62%.
[0039] Its structural identification data are as follows:
[0040] 1 HNMR (300MHz, CDCl 3 ), δ (ppm): 8.65 (1H, d), 8.37 (1H, dd), 7.73 (1H, d), 7.64 (1H, s), 4.48 (2H, q), 1.46 (3H, t).
Embodiment 3
[0042] Preparation of compound III (compound IIb is the compound in which X is Br in compound II)
[0043]
[0044] Add 23.1 g (0.10 mol) of 2-hydroxy-5-nitrobenzyl bromide and 18.3 g (0.11 mol) of triethyl phosphite into the reaction flask, stir and raise the temperature to 100° C., keep the temperature for 3.5 hours, and cool for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com