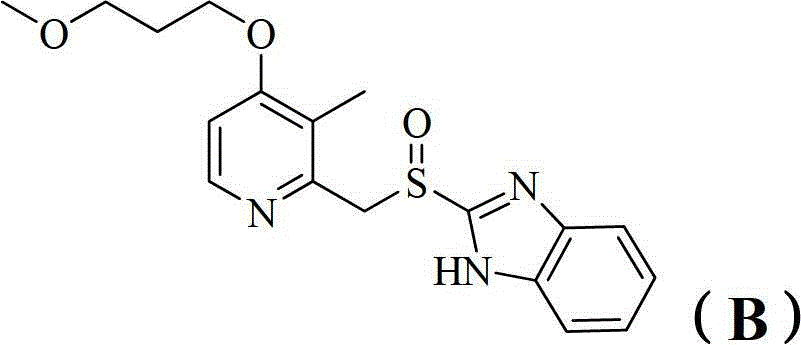
Benzimidazole derivatives and pharmaceutical compositions and uses thereof
A technology of imidazoles and compounds, applied in the field of benzimidazole derivatives, can solve the problems of long strongest time and large individual differences in pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Example 1: 2-[[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl]- Preparation of 6,7-dihydro-3H-benzofuro[5,6-d]imidazole (compound 1)
[0177] Step 1: Preparation of 5-nitro-2,3-dihydrobenzofuran
[0178]
[0179] 2,3-Dihydrobenzofuran (5 g, 41.6 mmol) was dissolved in 35 mL of acetic acid, and 1 / 4 HNO was added dropwise 3 (0.9 mL, 45.4 mmol). It was heated to 70°C at the beginning of the reaction, and then the remaining HNO 3 join in. After half an hour the reaction was cooled, added to ice water, then washed with Na 2 CO 3 neutralize. The aqueous layer was extracted with ethyl acetate. The combined organic layers were dried, concentrated in vacuo and purified by column chromatography to give the product (1 g, 14.6%).
[0180] Step 2: Preparation of 5-amino-2,3-dihydrobenzofuran
[0181]
[0182] The product obtained in Step 1 (1 g, 6.1 mmol), Raney Ni (0.1 g) and MeOH (10 mL) were hydrogenated at room temperature under a hydrogen pressure of ...
Embodiment 2
[0210] Example 2: 2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]- Preparation of 6,7-dihydro-3H-benzofuro[5,6-d]imidazole (compound 2)
[0211] Steps 1-7 are carried out with reference to Steps 1-7 of Example 1.
[0212] Step 8: 2-[(4-Methoxy-3,5-dimethylpyridin-2-yl)methylthio]-6,7-dihydro-3H-benzofuro[5,6-d ] The preparation of imidazole
[0213]
[0214] Referring to Step 8 of Example 1, throw 6-mercapto-7H-2,3-dihydrobenzofuro[5,6-d]imidazole (1.5g, 7.8mmol), NaOH (0.78g, 19.5mmol), acetone ( 10mL), water (10mL), 2-(chloromethyl)-4-methoxy-3,5-lutidine (1.45g, 7.8mmol), and the product (1.51g, 56.7%) was obtained.
[0215] Step 9: Preparation of Compound 2
[0216]
[0217] Referring to step 9 of Example 1, cast 2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-6,7-dihydro-3H-benzofuran[ 5,6-d] imidazole (1.42g, 4.16mmol), dichloromethane (20mL), m-CPBA (0.72g, 4.16mmol) to give the product (1.38g, 92.9%).
[0218] 1 H-NMR (DMSO, 600MHz): δ2.21 (6H...
Embodiment 3
[0223] Example 3: 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]- Preparation of 6,7-dihydro-3H-benzofuro[5,6-d]imidazole (compound 3)
[0224] Steps 1-7 are carried out with reference to Steps 1-7 of Example 1.
[0225] Step 8: 2-[[3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylthio]-6,7-dihydro-3H-benzo Preparation of furo[5,6-d]imidazole
[0226]
[0227] Referring to Step 8 of Example 1, throw 6-mercapto-7H-2,3-dihydrobenzofuro[5,6-d]imidazole (1.5g, 7.8mmol), NaOH (0.78g, 19.5mmol), acetone ( 10mL), water (10mL), 2-(chloromethyl)-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (1.87g, 7.8mmol), the product (1.63g , 52.8%).
[0228] Step 9: Preparation of compound 3
[0229]
[0230] Referring to step 9 of Example 1, cast 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylthio]-6,7-dihydro -3H-benzofuro[5,6-d]imidazole (1.64g, 4.16mmol), dichloromethane (20mL), m-CPBA (0.72g, 4.16mmol) to give the product (1.55g, 90.7%).
[0231] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com