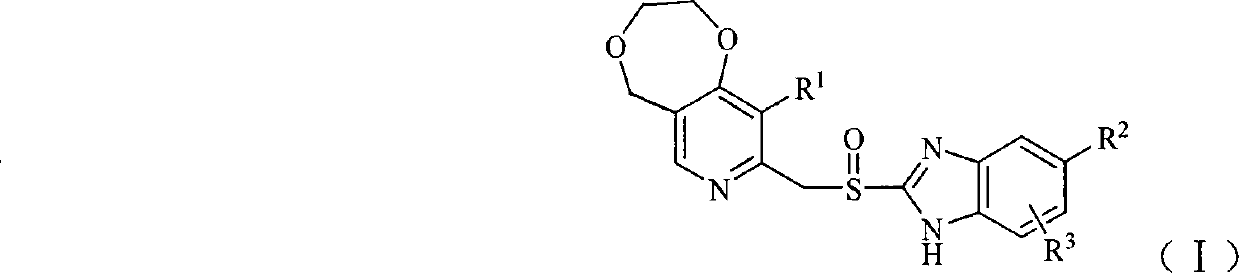
Sulfhydryl benzimidazole derivative containing dioxepane-pyridine
A benzimidazole and alkyl technology, applied in the application field of medicine, can solve the problems of insufficient drug effect, slow onset time, affecting drug effect and pharmacokinetic parameters, etc.
Active Publication Date: 2010-11-17
SHANDONG XUANZHU PHARMA TECH CO LTD
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the onset time of these drugs is slow, and the drug effect is not strong enough. It takes several doses (that is, a few days) to achieve the maximum acid-suppressing effect, and it may not be able to stably suppress acid for 24 hours. The time of taking the drug and eating may affect the effect of the drug. Pharmacokinetic parameters, individual differences in pharmacokinetics, and significant interactions with other drugs
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention belongs to the pharmaceutical technical field and specifically relates to hydrosulphonyl benzoglioxaline derivatives which are shown in general formula (I) and contain dioxo heptane pyridine, isomers and pharmaceutically acceptable salts thereof, wherein, R<1>, R<2> and R<3> are defined as in the specification. The invention also relates to preparation methods of the compounds, drugcompositions containing the compounds and the application of the compounds in preparing drugs for preventing and / or treating digestive ulcer.
Description
1. Technical field The present invention belongs to the technical field of medicine, and specifically relates to mercaptobenzimidazole derivatives containing dioxepane pyridine, its isomers and pharmaceutically acceptable salts thereof, preparation methods of these compounds, and medicines containing these compounds The composition and the application of these compounds in the preparation of medicines for treating and / or preventing peptic ulcer. 2. Background technology Digestive system diseases are one of the common frequently-occurring diseases, among which the incidence of ulcer disease accounts for about 10% to 12% of the total population. The initial treatment method mainly used antacids (such as sodium bicarbonate, aluminum hydroxide, etc.) to neutralize gastric acid to reduce symptoms. After the 1970s, with H 2 The discovery of gastric acid secretion inhibitors such as receptor blockers and proton pump inhibitors opened up a new era in the treatment of peptic ulcers. Th...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D491/04A61K31/4353A61P1/04
Inventor 黄振华
Owner SHANDONG XUANZHU PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com