Sustained release tablet containing theobromine
A technology of theobromine and sustained-release base, which is applied in the direction of medical preparations containing active ingredients, pill delivery, drug combination, etc., which can solve the problems of patient inconvenience, prolonged treatment time, and inability to maintain drug concentration, etc., to improve cough The effect of symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
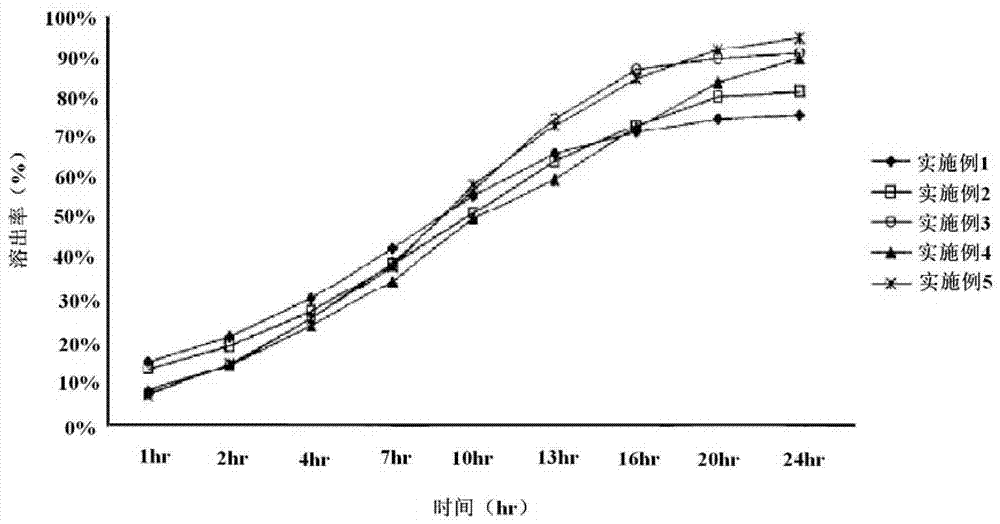
Embodiment 1
[0028] Table 1 (unit: weight %)
[0029]
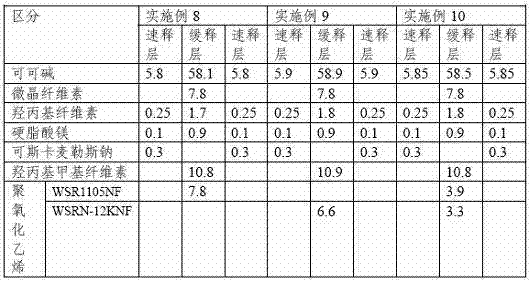
[0030] Table 2 (unit: weight %)
[0031]
[0032] Table 3 (unit: weight %)
[0033]
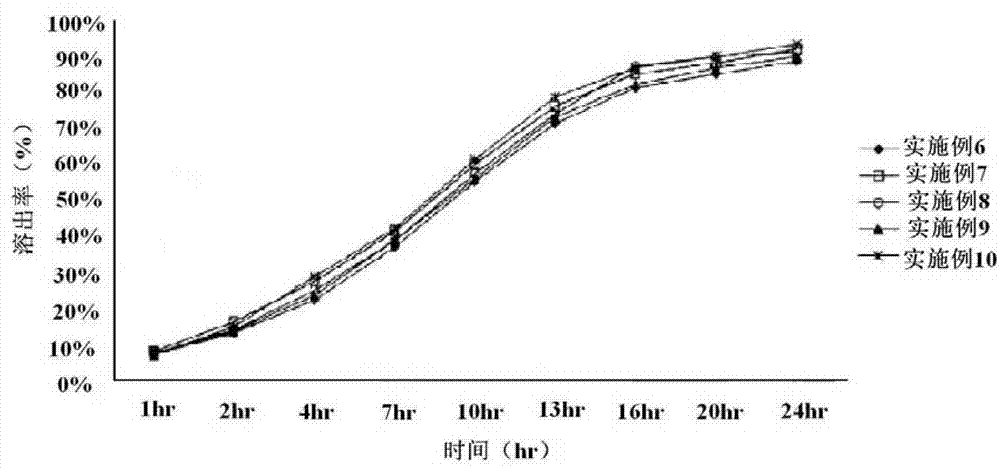
[0034] According to the structural proportions of the immediate-release layer recorded in the above Tables 1 to 3, the active substance "theobromine" and the excipient "microcrystalline cellulose" were mixed together, and hydroxypropyl cellulose was added here. After the granulation process, the above-mentioned granules are dried, and then coscamiles sodium and magnesium stearate are mixed here to produce an immediate-release layer compound.
[0035]Then, according to the structural proportions of the slow-release layer recorded in the above Tables 1 to 3, the active substance "theobromine" is mixed with microcrystalline cellulose and hydroxypropyl methylcellulose, and then added here The binding solution composed of propyl cellulose, after the wet granulation process, the above-mentioned granules are dried. After mixing polyethylene oxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com