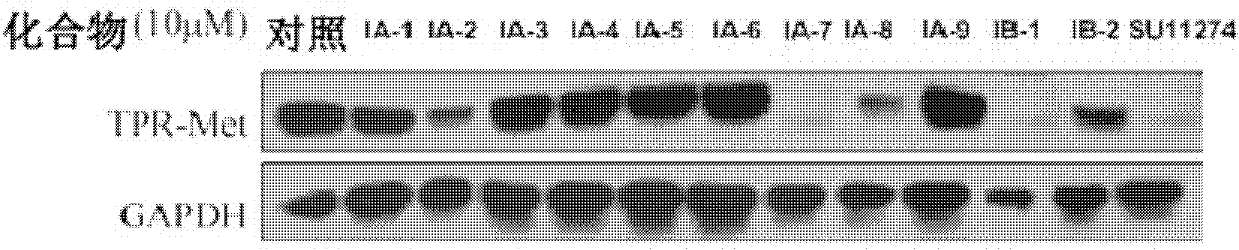
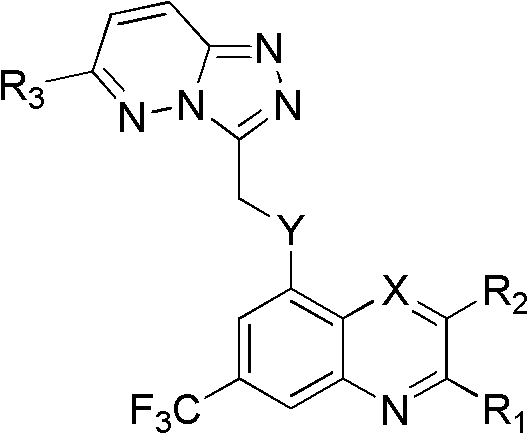
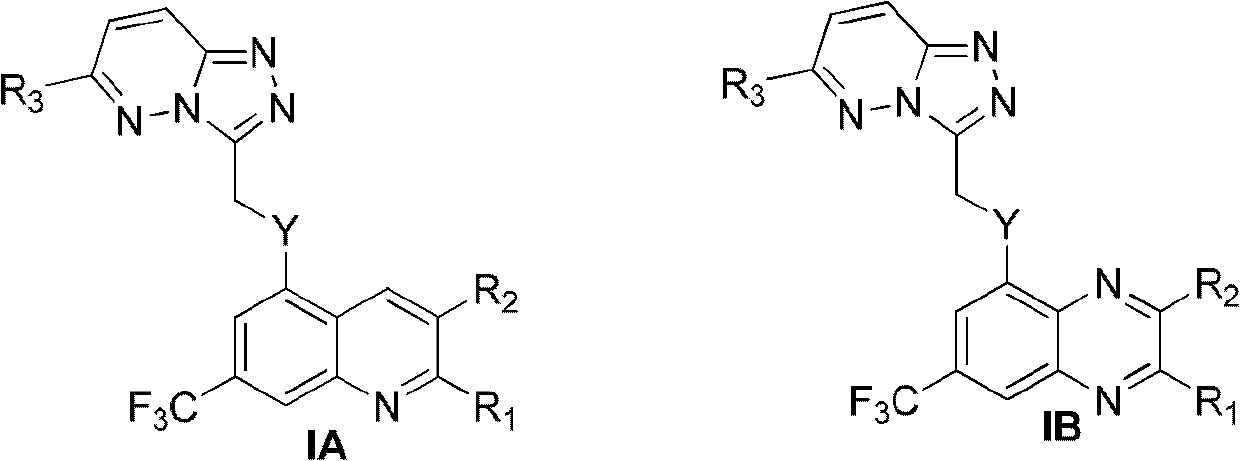
Trifluoro methyl substituted quinoline or quinoxaline compound and preparation method thereof, and pharmaceutical composition containing the compound and purpose thereof
The technology of a compound and quinoxaline is applied in the fields of trifluoromethyl-substituted quinoline or quinoxaline compounds, their preparation, pharmaceutical compositions containing the compound and their uses, and can solve the problem of nitro functional group mutagenesis, Carcinogenicity, poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0064] Preparation of Example 1 Compound IA-1
[0065] 1.1 Synthesis of intermediate 13
[0066]
[0067] Compound synthesis, reagents and conditions: i) potassium nitrate, concentrated sulfuric acid; ii) ammonium sulfide, ethanol, reflux; iii) a sodium nitrite, hydrobromic acid; b cuprous bromide, hydrobromic acid, reflux; iv) a N,N-dimethylformamide dimethyl acetal, N,N-dimethylformamide, reflux; b sodium periodate, N,N-dimethylformamide / water; v) iron powder, chlorine ammonium chloride, ethanol / water; vi)12, sodium hydroxide, ethanol, reflux.
[0068] 1.1.1 Synthesis of Compound 7
[0069] 4-Methyltrifluorotoluene (9.3 g, 58 mmol) was added into a round bottom flask containing 120 mL of concentrated sulfuric acid, and potassium nitrate (14.6 g, 145 mmol) was added with stirring and stirred at room temperature for 16 hours. The reaction solution was poured into ice water (300 mL). The aqueous phase was extracted three times with ethyl acetate, the organic phase was wa...
preparation Embodiment 2
[0092] Preparation of Example 2 Compound IA-2
[0093]
[0094] 2.1 Synthesis of intermediate 18
[0095] Intermediate 18 was synthesized in the same way as Intermediate 17 except that furan-2-boronic acid was used instead of phenylboronic acid.
[0096]
[0097] 1 H-NMR (300MHz, CDCl 3 ): δ8.10(d, J=9.9Hz, 1H), 7.65(s, 1H), 7.56(d, J=9.9Hz, 1H), 7.23(d, J=3.0Hz, 1H), 6.62(s , 1H), 5.45(s, 1H), 4.95(d, J=3.0Hz, 2H), 1.45(s, 9H).
[0098]
[0099] 1 H-NMR (300MHz, CDCl 3 ): δ8.18(d, J=9.6Hz, 1H), 7.65(d, J=1.2Hz, 1H), 7.56(d, J=9.6Hz, 1H), 7.20(d, J=5.2Hz, 1H ), 6.62(m, 1H), 4.44(s, 2H).
[0100] 2.2 Preparation of compound IA-2
[0101] Compound IA-2 was synthesized in the same manner as Compound IA-1 except that Intermediate 18 was used instead of Intermediate 17.
[0102] 1 H NMR (300MHz, CDCl 3 )δ8.74(d, J=2.7Hz, 1H), 8.09(d, J=8.4Hz, 1H), 7.68(s, 2H), 7.57(d, J=8.1Hz, 1H), 7.35(d, J=2.1Hz, 1H), 7.25(s, 1H), 6.95(s, 1H), 6.65(dd, J=3.6, 1.5Hz, 1H), 5.94...
preparation Embodiment 3
[0103] Preparation of Example 3 Compound IA-3
[0104]
[0105] 3.1 Synthesis of Intermediate 19
[0106] Intermediate 19 was synthesized in the same way as Intermediate 17 except that thiophene-2-boronic acid was used instead of phenylboronic acid.
[0107]
[0108] 1 H-NMR (300MHz, CDCl 3 ): δ8.08(d, J=9.6Hz, 1H), 7.70(d, J=4.8Hz, 1H), 7.54(m, 2H), 7.17(m, 1H), 5.49(s, 1H), 4.95 (d, J=5.1Hz, 2H), 1.46(s, 9H).
[0109]
[0110] 1 H-NMR (300MHz, CDCl 3 ): δ8.08(d, J=9.6Hz, 1H), 7.68(m, 1H), 7.54(m, 1H), 7.50(d, J=9.6Hz, 1H), 7.17(m, 1H), 4.45 (s, 2H).
[0111] 3.2 Preparation of compound IA-3
[0112] Compound IA-3 was synthesized in the same manner as Compound IA-1, except that Intermediate 19 was used instead of Intermediate 17.
[0113] 1 H NMR (300MHz, CDCl3) δ8.77(d, J=2.7Hz, 1H), 8.11(d, J=9.6Hz, 1H), 7.72(d, J=3.9Hz, 2H), 7.58(d, J =5.4Hz, 1H), 7.40(d, J=9.6Hz, 1H), 7.35(d, J=2.7Hz, 1H), 7.20(dd, J=4.8, 3.9Hz, 1H), 6.96(s, 1H ), 5.74(t, J=6.3Hz, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com