N-acetyl-l-cysteine for the treatment of endometriosis
A technology for endometriosis and cysteine, applied in the field of pharmaceutical compositions containing N-acetyl-L-cysteine, can solve the clinical results of endometriosis without a proposed dosage regimen Questions such as undecided
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
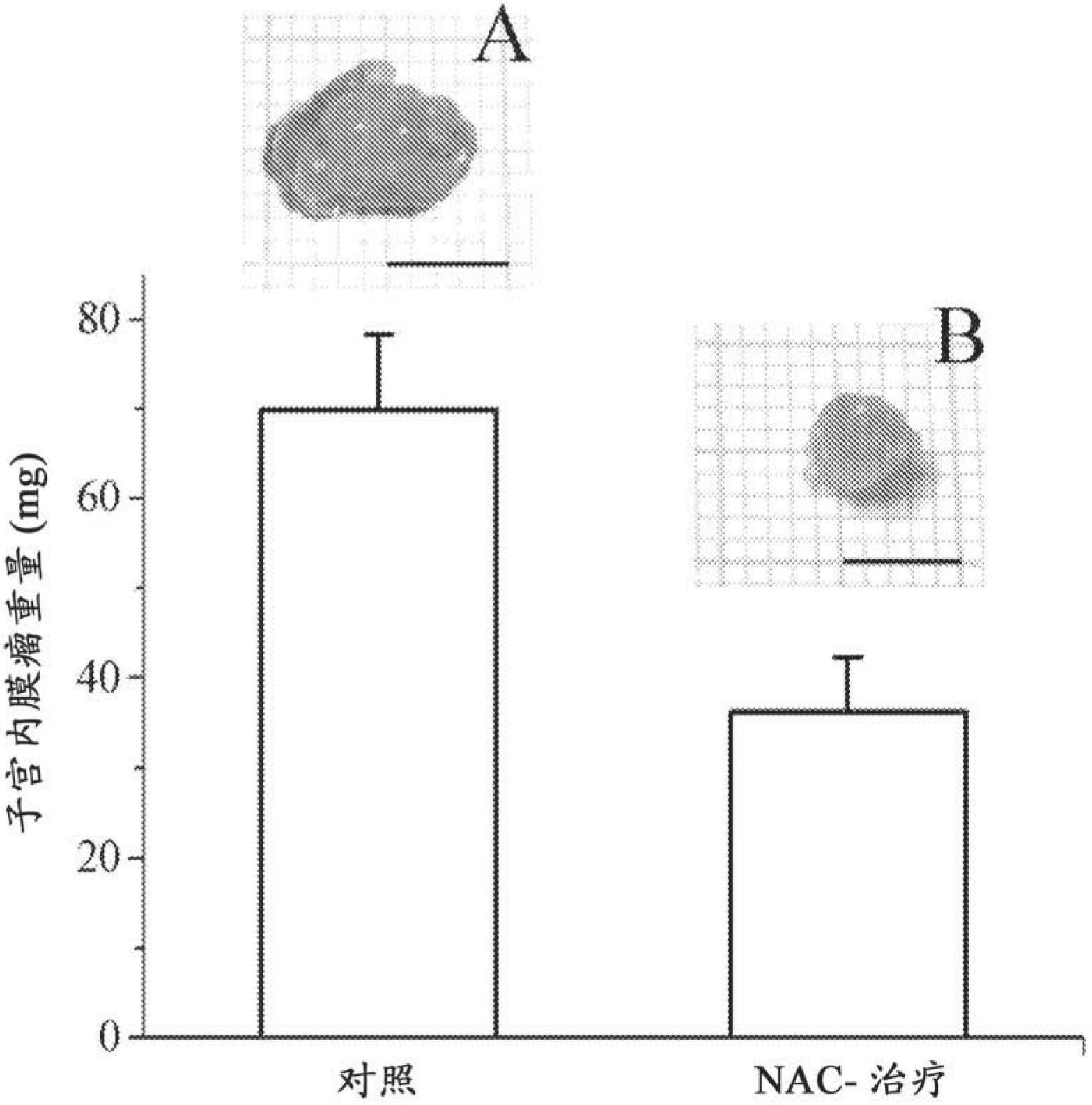
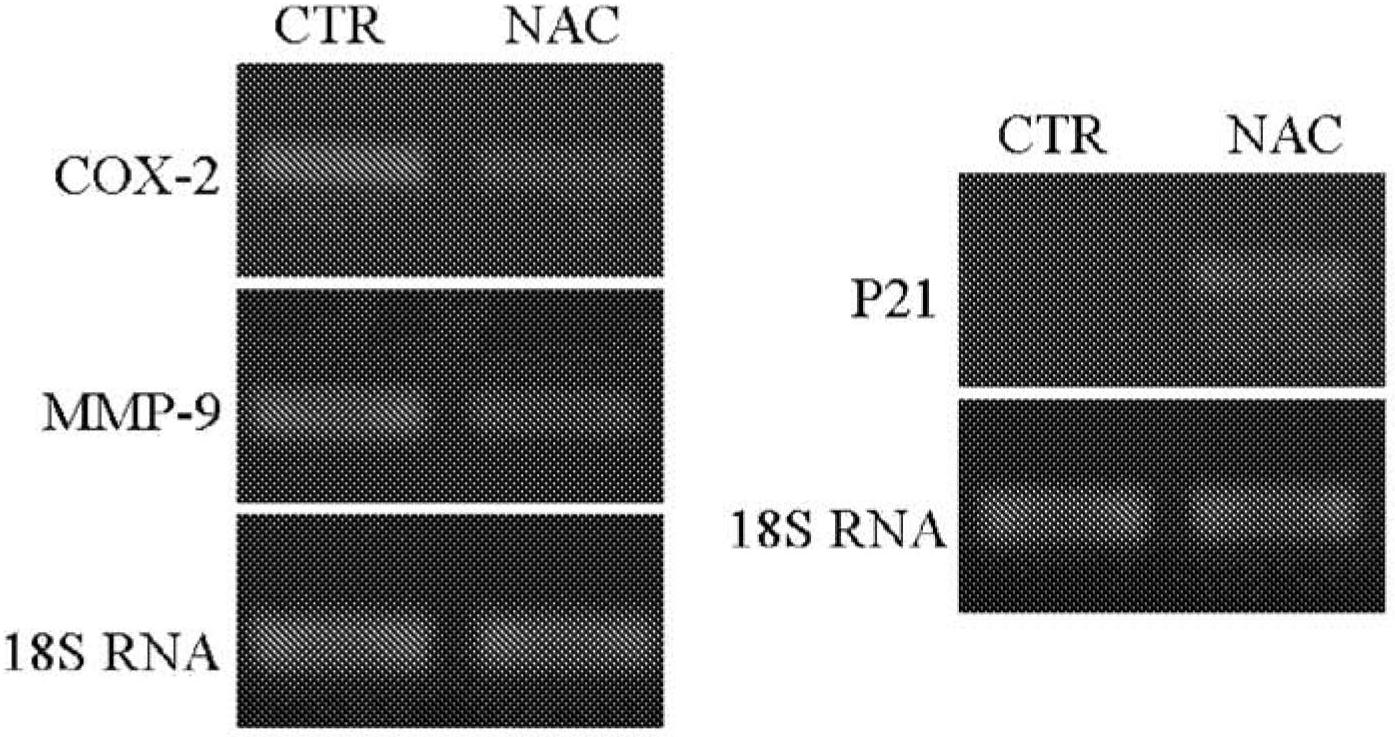
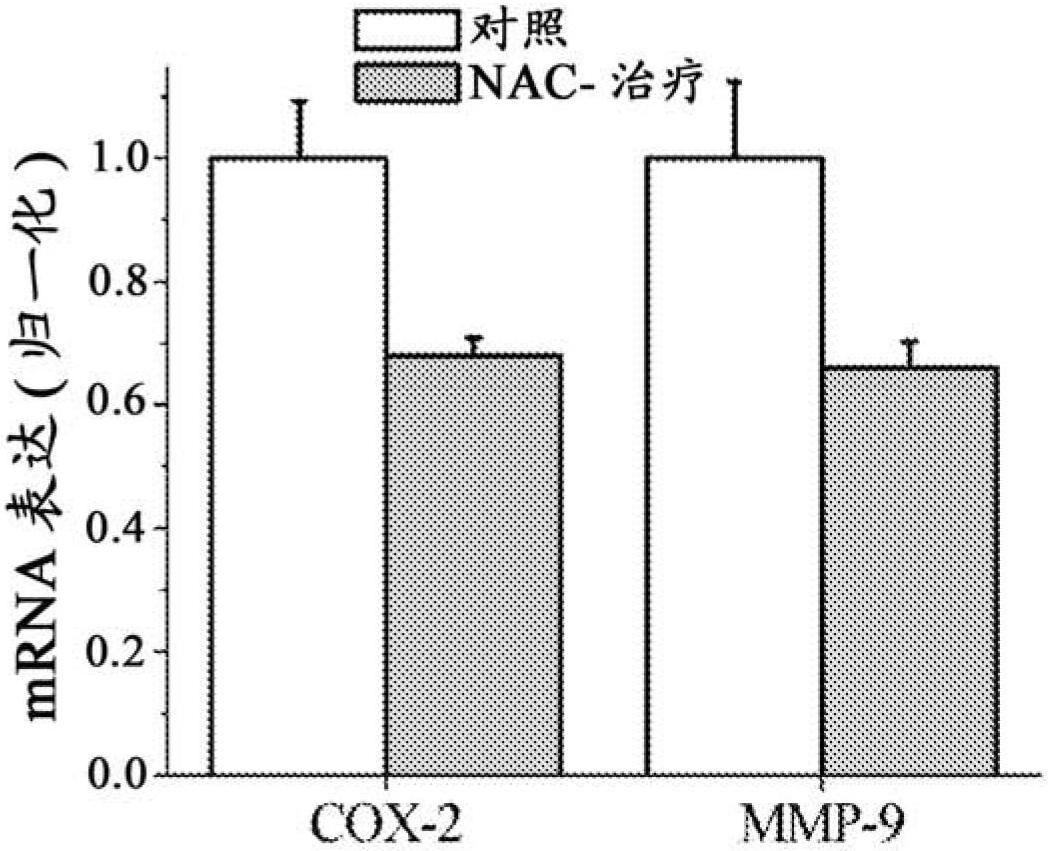
[0094] Example 1. Effects in a mouse model of endometriosis
[0095] Materials and methods
[0096] Material. All chemicals were from Sigma-Aldrich, Milan, Italy unless otherwise noted.
[0097] animal. Thirty-six female, 6- to 8-week-old BALB / C mice were purchased from Charles River Italia (Calco, Italy). Feed pellets (concentrated standard diet; Mucedola, Milan, Italy) and water were provided ad libitum. Animals were housed on a 16 / 8 hour light / dark cycle under controlled conditions. Before any invasive procedure, mice were anesthetized by intraperitoneal injection of 0.4 ml of a physiological solution containing 2.5% AVERTIN.
[0098] Induction of endometriosis. Induction of endometriosis was performed by the method published by Somigliana et al., Hum Reprod 1999 Dec; 14(12):2944-50. Perform surgical interventions under clean conditions. Briefly, the uterine horns of syngenic mice were removed through a small incision in the midline of the peritoneal space below the...
Embodiment 2
[0121] Example 2. Preclinical research on the effect of NAC in treating endometriosis
[0122] Research purposes. This pilot clinical study was designed to use NAC instead of other therapies for the treatment of women with endometriosis. The only possible additional treatment is analgesics for pain relief when needed.
[0123] Conditions for patient enrollment. Women with an initial diagnosis of endometriosis based on disease-based pain and ovarian ultrasound evidence, and patients with pain and / or ovarian endometrioma recurrence after laparoscopic treatment were enrolled in the study.
[0124] Before treatment, the intensity of pain symptoms was measured by means of a 10-point visual analog scale (VAS), and the characteristics of ovarian endometriomas were measured by ultrasound. During NAC treatment, other treatments were excluded except for pain management with analgesics when needed.
[0125] patient. From February 2008 to July 2010, 64 women diagnosed with ovarian en...
Embodiment 3
[0136] Example 3: Effect of Pulsed, Intermittent and Daily NAC Treatment on Experimentally Induced Endometriosis: A Pilot Study in Rats of Vaginal Hyperresponsiveness as a Sign of Pain
[0137] Materials and methods
[0138] animal. Sixteen virgin female Sprague-Dawley rats were used. They weighed 200-230 g at the beginning of the study and were housed in plastic cages with litter in a controlled room. Rats had ad libitum access to water and chow, and were kept on a 12-h light / dark cycle, with lighting starting at 07:00. Reproductive status (proestrus, estrus, postestrus, and diestrus) was determined by daily vaginal douching.
[0139] Experimental models and preoperative training. Prior to induction of endometriosis, rats were trained to develop an escape response to terminal vaginal dilatation by an inflatable latex balloon. A small, deflated latex balloon is tied to a thin, lubricated catheter and inserted into the midvaginal cavity. The balloon is inflated with air a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com