Valsartan sustained release tablet and preparation method thereof
A technology for valsartan and tansan, which is applied in the field of sustained-release matrix tablets containing the active ingredient valsartan and its preparation, and can solve problems such as blood pressure fluctuations, large fluctuations in blood drug concentration, and difficulty in process realization
Inactive Publication Date: 2012-08-22
SHANDONG INST OF PHARMA IND
View PDF3 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
It will be very difficult to prepare a drug with a short half-life for 24-hour administration
[0021] The half-life of common preparations of valsartan is about 6 to 9 hours. Although it is used once a day, that is to say, its therapeutic index is relatively wide, but this will cause excessive fluctuations in blood drug concentration, and the blood drug concentration is not stable enough. In terms of therapeutic effect, it is unable to lower blood pressure more effectively and steadily, resulting in large fluctuations in blood pressure. Therefore, the development of valsartan sustained-release tablets is considered.
[0022] Patent CN101951902A provides a solid oral dosage form of valsartan and a method for preparing the preparation, which mentions a MR tablet, which is granulated by melting method, and finally pressed into an oval sustained-release tablet, but the melting method granulation equipment is not available in China. Not many, the process is difficult to realize, and the reproducibility is poor
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a preparation method of a valsartan sustained release matrix tablet. Particular prescription components (g / g) of a carbazochrome sodium sulfonate trihydrate sustained release tablet is 20-50% of valsartan, 8-20% of framework materials, 35-75% of diluent, 0-5% of binding agent and 1-4% of lubricating agent. The preparation method adopts full powder direct compression or adopts a wet granulation to achieve compression after dryimng.
Description
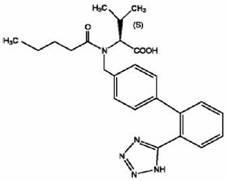
technical field [0001] The invention belongs to the technical field of drug sustained-release preparations, and in particular relates to a sustained-release matrix tablet containing an active ingredient valsartan and a preparation method thereof. Background technique [0002] The chemical name of valsartan is N-(1-pentanoyl)-N-[4-[2-(1H-tetrazol-5-yl)phenyl]benzyl]-L-valine, and its structural formula is as follows: [0003] [0004] The English instructions issued by the FDA: The raw material of valsartan is white to off-white fine powder, soluble in methanol and ethanol, slightly soluble in water. [0005] The import standard of raw materials is: this product is white or off-white fine powder, hygroscopic. This product is dissolved in methanol and slightly soluble in water. [0006] Chinese Pharmacopoeia 2010 Edition: This product is white crystal or white, off-white powder; hygroscopic. This product is very soluble in ethanol, easily soluble in methanol, slightly s...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/22A61K31/41A61P9/12
Inventor 陈修毅曹冲
Owner SHANDONG INST OF PHARMA IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com