Processes for the preparation of deferasirox, and deferasirox polymorphs
一种地拉罗司、结晶的技术,应用在植物学设备和方法、药物组合、动物驱避剂等方向,能够解决繁多步骤、复杂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0313] Example 1 - Method A
[0314] o-Trimethylsiloxybenzonitrile (IV, X=SiMe 3 ) as described in British Patent GB 1330265 or Tetrahedron Letters, 1986, 27(3):347-348.
[0315] 2-(Methoxymethyl)oxybenzonitrile (IV, X=MOM) was prepared according to Tetrahedron, 2003, 59:5831-5836.
[0316] 2-Acetoxybenzonitrile (IV, X=Ac) was prepared as described in Bulletin de la Siete Chimique de France, 1958, 185-187.
[0317] General preparation of compounds of formula II:
[0318]Under an inert atmosphere, 16.0 g (0.2 mol) of sulfur trioxide was added dropwise at 0-3 ° C to treat dichloride in 75 ml of dichloroethane. 17.6g (0.2mol) of alkane, accompanied by cooling and stirring, thus generating di Alkane-sulfur trioxide complex slurry. Nitrile IV (0.4 mol) in 100 ml of dichloroethane was then added to the reaction mixture and the temperature was allowed to rise slowly to room temperature. Reaction monitoring was performed by TLC. After the reaction was complete, the solid wa...
Embodiment 2
[0321] Example 2 - Method B :
[0322] Preparation of salicyloyl chloride
[0323] Salicylic acid (5.0g, 36.2mmol) was suspended in dry hexane (40ml) and thionyl chloride (4.52g, 38mmol) was added under nitrogen atmosphere followed by a drop of pyridine. The mixture was refluxed for 2 hours with stirring. The clear yellow solution was cooled and concentrated in vacuo to give a thick oil which was used in the next step.
[0324] Preparation of 2-hydroxy-N-(2-hydroxybenzyl)benzamide [bis(salicyl)imide] (4)
[0325] 6 salicyloyl chloride+5(Me 3 Si) 2 NH→3 bis(salicyl)imide+4Me 3 SiCl+2NH 4 Cl
[0326] Salicyloyl chloride prepared above was dissolved in dry toluene (10ml) and added dropwise to a solution of hexamethyldisilazane (4.954g, 30.8mmol) in dry toluene (20ml). The mixture was stirred at 0-10 °C under nitrogen atmosphere for 1 hour. The mixture was then filtered, and the filtrate was concentrated under reduced pressure. Ethanol was added to the residue, and...
Embodiment 3
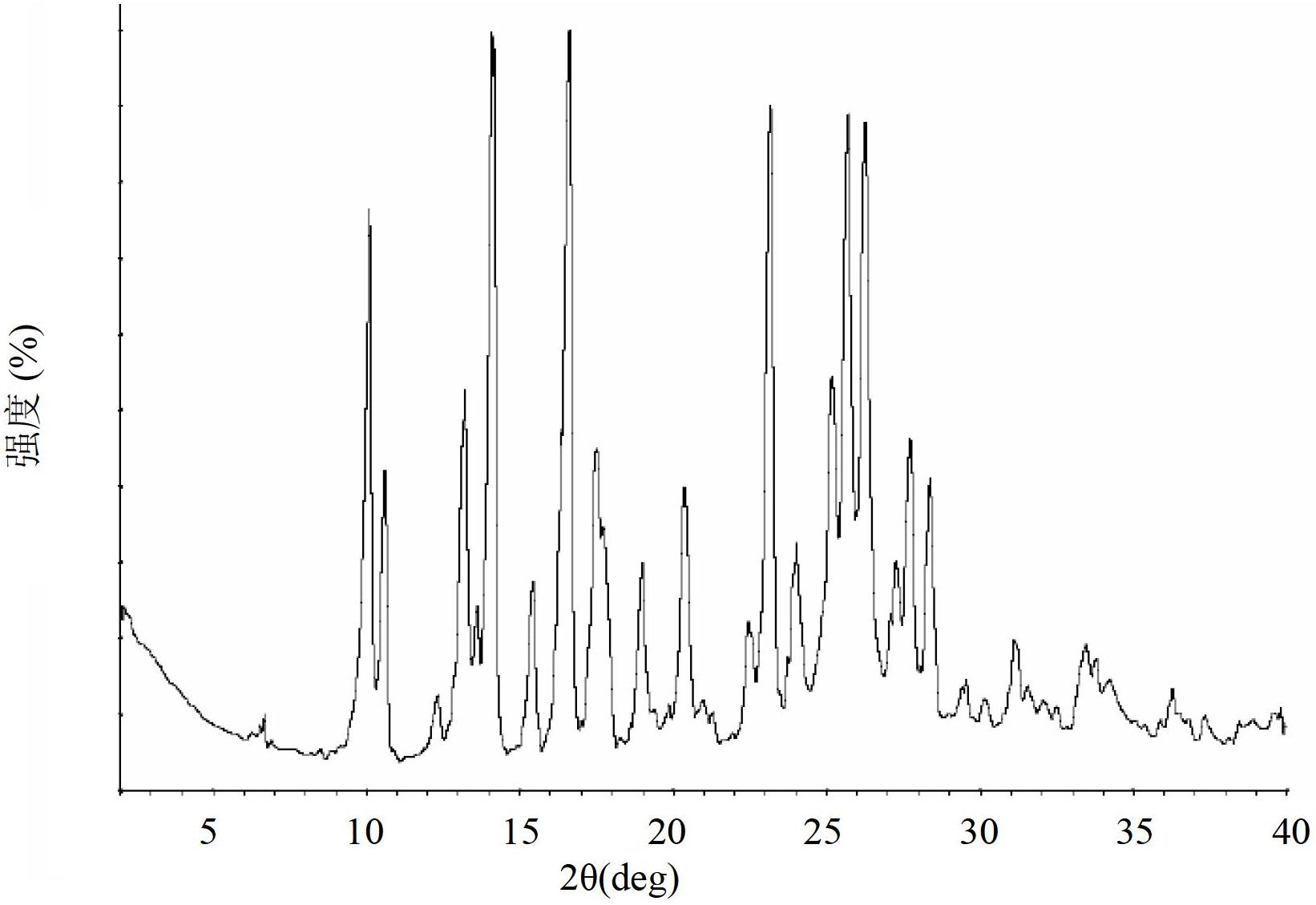
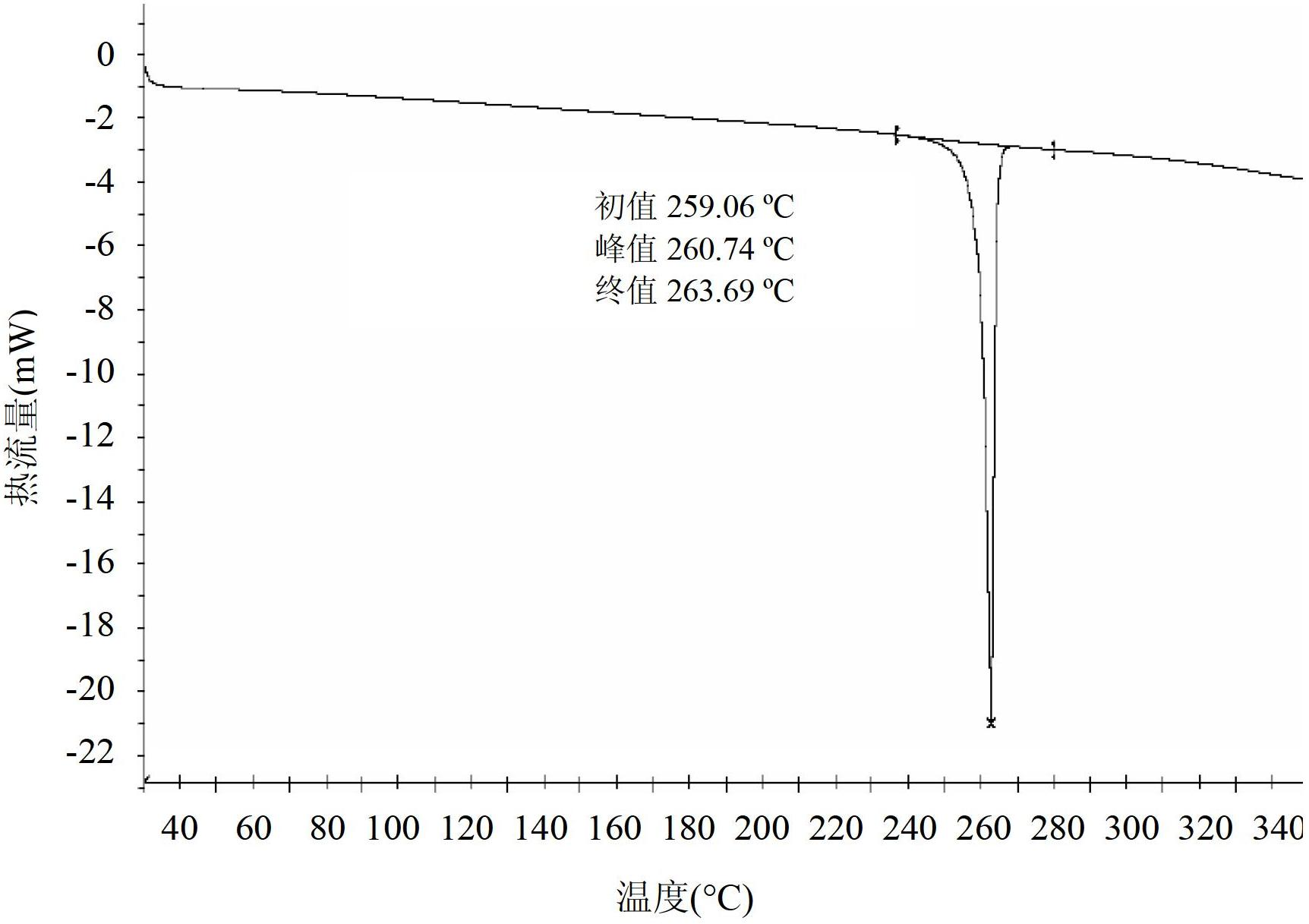
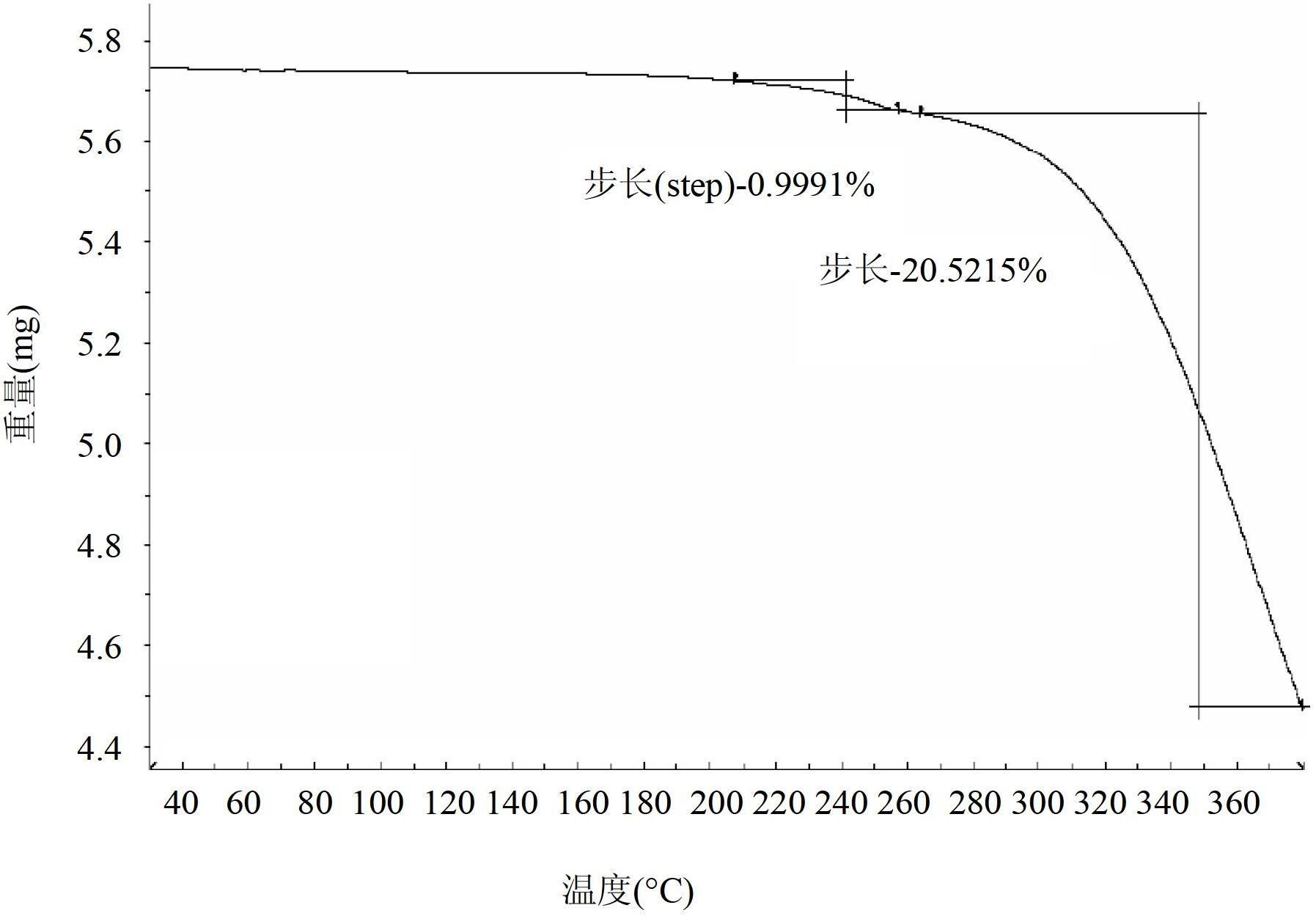
[0335] Example 3 - General preparation of polymorphs of deferasirox
[0336] 1. Reagents
[0337] Acetonitrile, HPLC grade, Sigma, Lot No.07278PH
[0338] Ethanol, HPLC grade, Sigma, Lot No. 11085CH
[0339] DMSO, HPLC grade, Sigma, Lot No.05737BH
[0340] Dichloromethane, Alfa Aesar, HPLC grade, Lot No. C27S008
[0341] Methanol, AR, SCRC, Lot No.T20090912
[0342] Ethanol acetate, AR, Yixing Secondary Chemical Company, Lot No.090607
[0343] MIBK, AR, SCRC, Lot No. T20080411
[0344] Isopropanol, AR, Sinopharm Chemical Reagent Co.Ltd, Lot No.T20090813
[0345] Acetone, AR, Sinopharm Chemical Reagent Co.Ltd, Lot No.090104
[0346] Toluene, AR, SCRC, Lot No.T20090603
[0347] tert-butyl methyl ether, HPLC grade, Fluka, Lot No. 1359496
[0348] THF, AR, Yixing Secondary Chemical, Lot No.090901
[0349] 1-Butanol, AR, SCRC, Lot No.T20080818
[0350] MEK, AR, SCRC, Lot No. T20090724
[0351] iPrOAc, AR, Shanghai Experimental Reagent Company, Lot No.20080410
[0352...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com