Synthesis method of supported silver iodide nanoparticle visible light photocatalyst
A nanoparticle, photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problems of easy deactivation, unstable visible light photocatalytic performance, etc. Easy to synthesize in large quantities and improve the effect of photocatalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
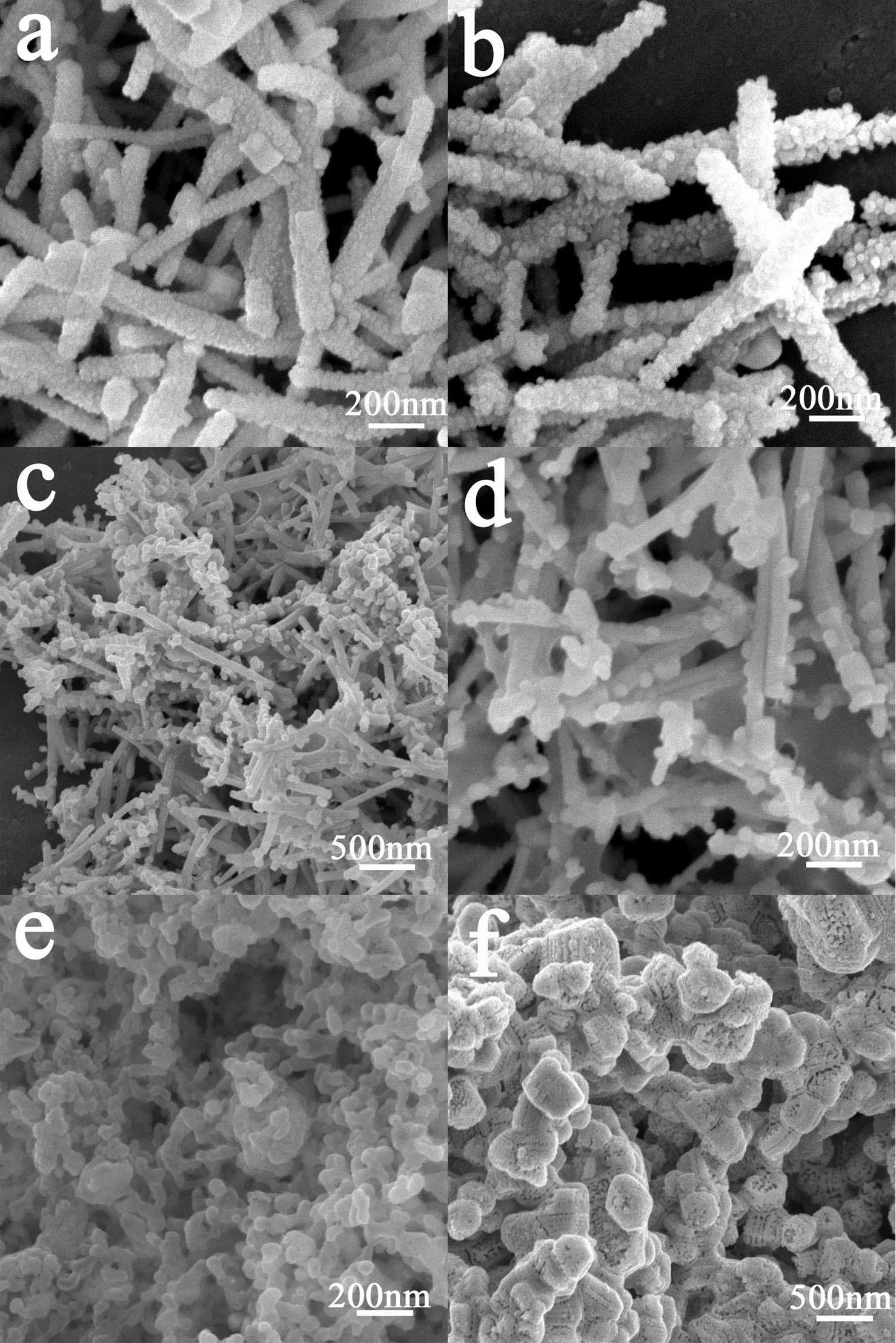
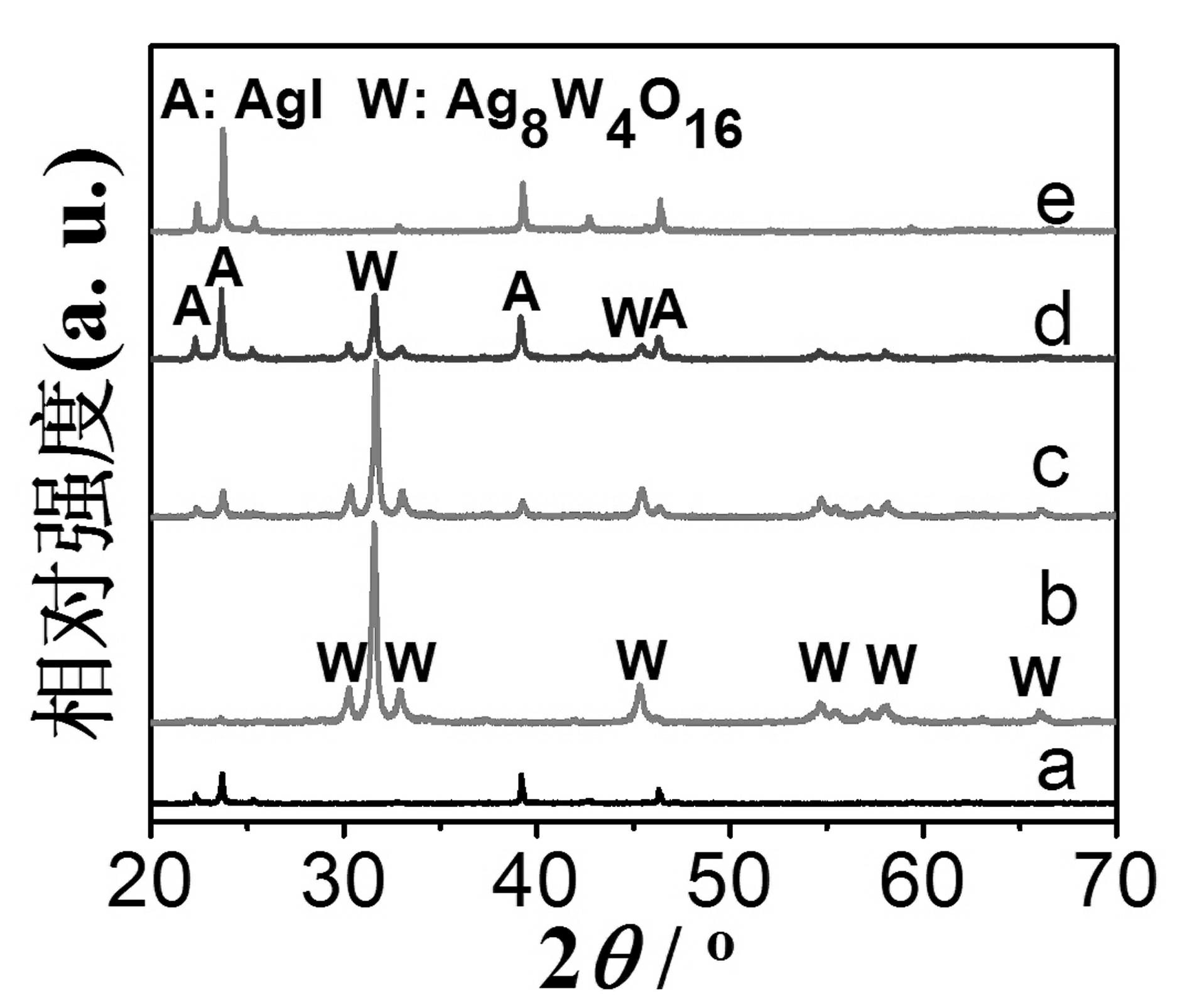
[0033] The synthesis method of supported silver iodide nanoparticles visible light photocatalyst comprises the following steps: 1) Preparation of silver tungstate nanorods: under room temperature, 40 milliliters of 0.01 moles / liter silver nitrate solution is added to 40 milliliters of 0.005 moles / liter tungsten NaCl solution, let it stand for 12 hours, and then washed the precipitate with deionized water for 3 times to obtain silver tungstate nanorods with a length of 0.5-2 microns and a diameter of 20-100 nanometers; 2) dissolving potassium iodide in deionized A uniform solution was formed in ionized water, and the pH value of the solution was adjusted to 6 with nitric acid; 3) Put 1 gram of Ag 2 WO 4 Add the nanorods to 25 ml of 0.002-0.2 mol / L potassium iodide solution, stir evenly, let stand in a dark room at room temperature for 2 hours, filter the yellow precipitate, wash the product 3 times with deionized water and absolute ethanol, and finally in Dry at 40° C. for 2 hou...
Embodiment 2
[0042] In order to test the influence of iodide ion concentration on the morphology and photocatalytic performance of supported silver iodide nanoparticles, the reaction conditions were the same as in Example 1 except that the concentration of potassium iodide was different. The results showed that when the concentrations of potassium iodide were 0.001, 0.002, 0.04, 0.1 and 0.2 mol / L, the degradation rate constants of AgI samples to methyl orange were 0.005, 0.013, 0.033, 0.018 and 0.001 min, respectively -1 . The main reason is that when the concentration of potassium iodide is 0.001 mol / L, only a small amount of AgI nanoparticles are loaded on the surface of silver tungstate nanorods due to the low concentration of iodide ions, and the photocatalytic degradation performance is low; when the concentration of potassium iodide is 0.002-0.1 mol / L, because AgI nanoparticles can be well loaded on the surface of silver tungstate nanorods, showing high photocatalytic activity (such ...
Embodiment 3
[0045] In order to examine the impact of the pH value of potassium iodide solution on the morphology and photocatalytic performance of loaded silver iodide nanoparticles, the concentration of potassium iodide solution (0.04 mol / liter), except that the pH value of potassium iodide solution is different, other reaction conditions are all with embodiment 1 same. The results show that when the pH value of the potassium iodide solution is 2, the silver tungstate nanorods can be converted into tungstic acid, which destroys the nanorod structure and cannot obtain the loaded AgI nanoparticle photocatalyst; when the pH value of the potassium iodide solution is 5 and 7, the morphology of the resulting supported silver iodide nanoparticle photocatalyst is similar to figure 1 and showed high catalytic performance for the degradation of methyl orange, with degradation rate constants of 0.034 and 0.032 min, respectively -1 ; When the pH value of the potassium iodide solution was 11, silver...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com