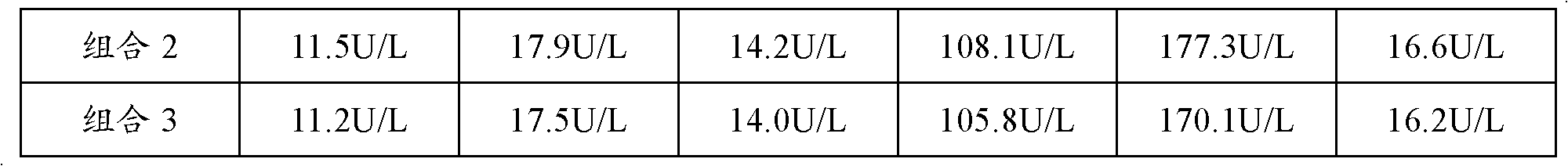Pharmaceutical composition of alanyl glutamine injection and compound amino acid injection
A technology of alanyl glutamine and compound amino acids, which is applied in the field of medicine and achieves the effects of reducing liver damage, saving costs, and facilitating procurement and dispensing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Combination Packaged Drugs
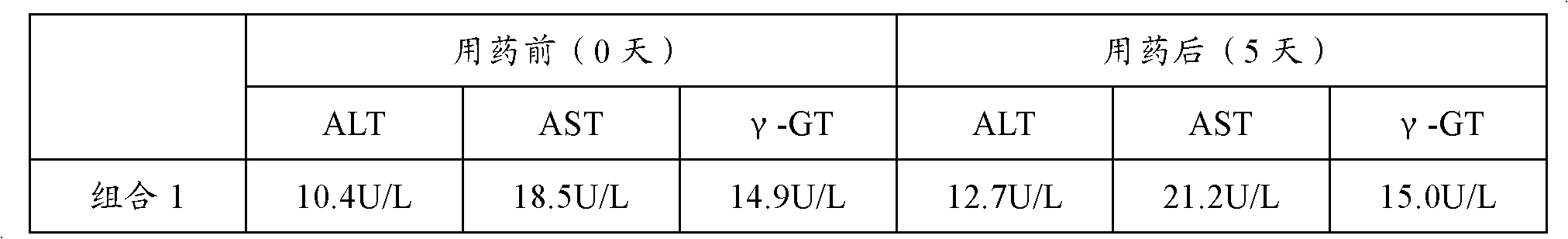
[0039] Combination: Alanyl Glutamine Injection 50ml: 10g and Compound Amino Acid Injection 250ml
[0040] making process:
[0041] 1. Preparation of Alanyl Glutamine Injection
[0042] (1) Concentrated formulation: add 15L of water for injection to the concentrated formulation tank, add 10000g of alanyl glutamine, stir to dissolve, add 500g of wet medicinal charcoal, stir for 30 minutes, filter for decarbonization, filter into dilute For the preparation tank, rinse the concentrated preparation tank with water for injection several times, and filter into the thin preparation tank;
[0043] (2) Dilute preparation: Add water for injection to 45L in the dilute preparation tank, adjust the pH value to 5.7 with 0.1mol / L sodium hydroxide solution, add 500g of wet medicinal charcoal, add water for injection to the full amount, and stir 30 minutes;
[0044] (3) intermediate product detection: detect the pH value of filtr...
Embodiment 2
[0054] Example 2 Preparation of Combination Packaged Drugs
[0055] Combination: Alanyl Glutamine Injection 100ml: 20g and Compound Amino Acid Injection 500ml
[0056] making process:
[0057] 1. Preparation of Alanyl Glutamine Injection
[0058] (1) Concentrated formulation: add 30L water for injection to the concentrated formulation tank, add 20000g of alanylglutamine, stir to dissolve, add 1000g of wet medicinal charcoal, stir for 30 minutes, filter for decarbonization, filter into dilute For the preparation tank, rinse the concentrated preparation tank with water for injection several times, and filter into the thin preparation tank;
[0059] (2) Dilute preparation: Add water for injection to 90L in the dilute preparation tank, adjust the pH value to 5.8 with 0.1mol / L sodium hydroxide solution, add 1000g of wet medicinal charcoal, add water for injection to the full amount, and stir 30 minutes;
[0060] (3) intermediate product detection: detect the pH value of filtr...
Embodiment 3
[0070] Example 3 Preparation of Combination Packaged Drugs
[0071] Combination: Alanyl Glutamine Injection 100ml: 20g and Compound Amino Acid Injection 500ml
[0072] making process:
[0073] 1. Preparation of Alanyl Glutamine Injection
[0074] (1) Concentrated formulation: add 30L water for injection to the concentrated formulation tank, add 20000g of alanylglutamine, stir to dissolve, add 1000g of wet medicinal charcoal, stir for 30 minutes, filter for decarbonization, filter into dilute For the preparation tank, rinse the concentrated preparation tank with water for injection several times, and filter into the thin preparation tank;
[0075] (2) Dilute preparation: add water for injection to 90L in the dilute preparation tank, adjust the pH value to 5.9 with 0.1mol / L sodium hydroxide solution, add 1000g of wet medicinal charcoal, add water for injection to the full amount, and stir 30 minutes;
[0076] (3) intermediate product detection: detect the pH value of filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com