Epoxy resin composition
A technology of epoxy resin and epoxy compound, which is applied in the direction of electrical components, circuits, transportation and packaging, and can solve the problems of inability to ensure storage stability at low temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
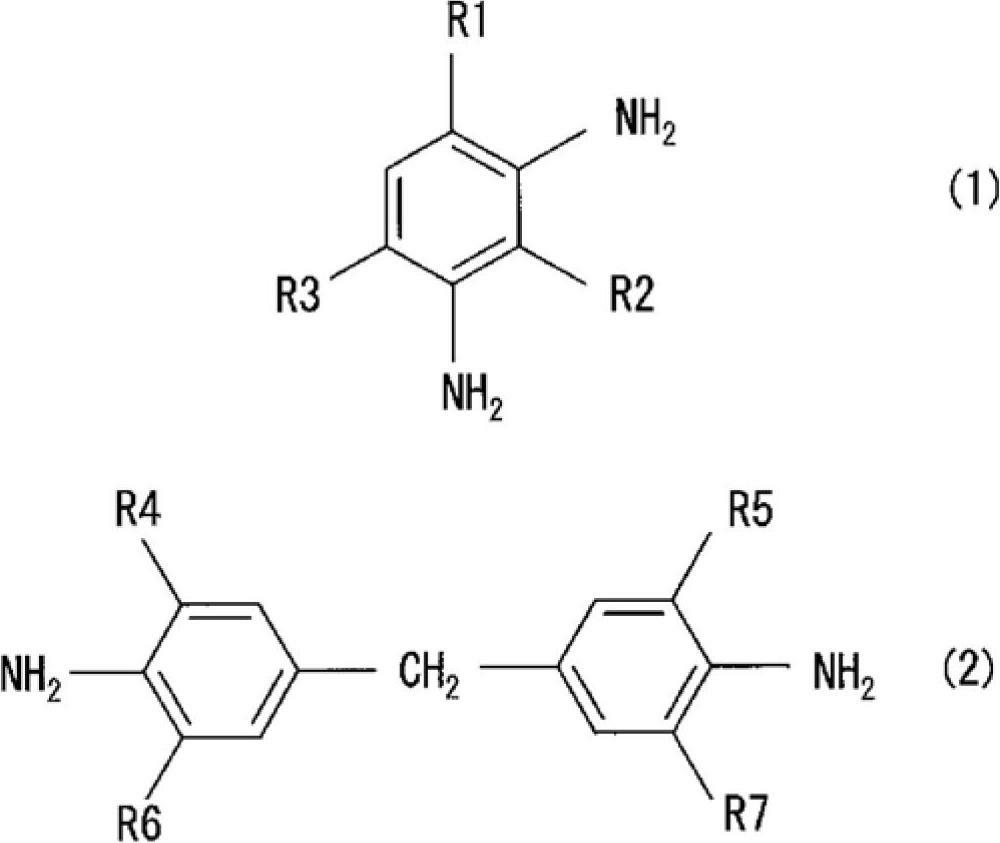
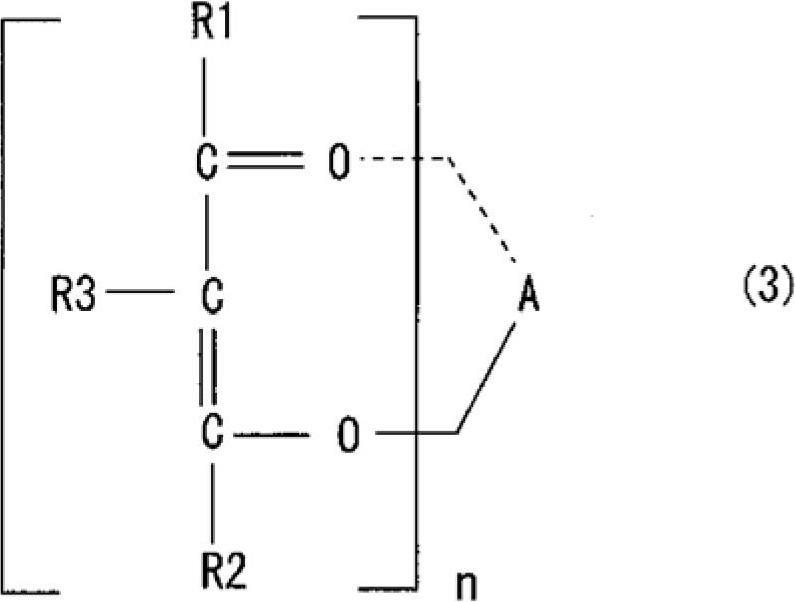
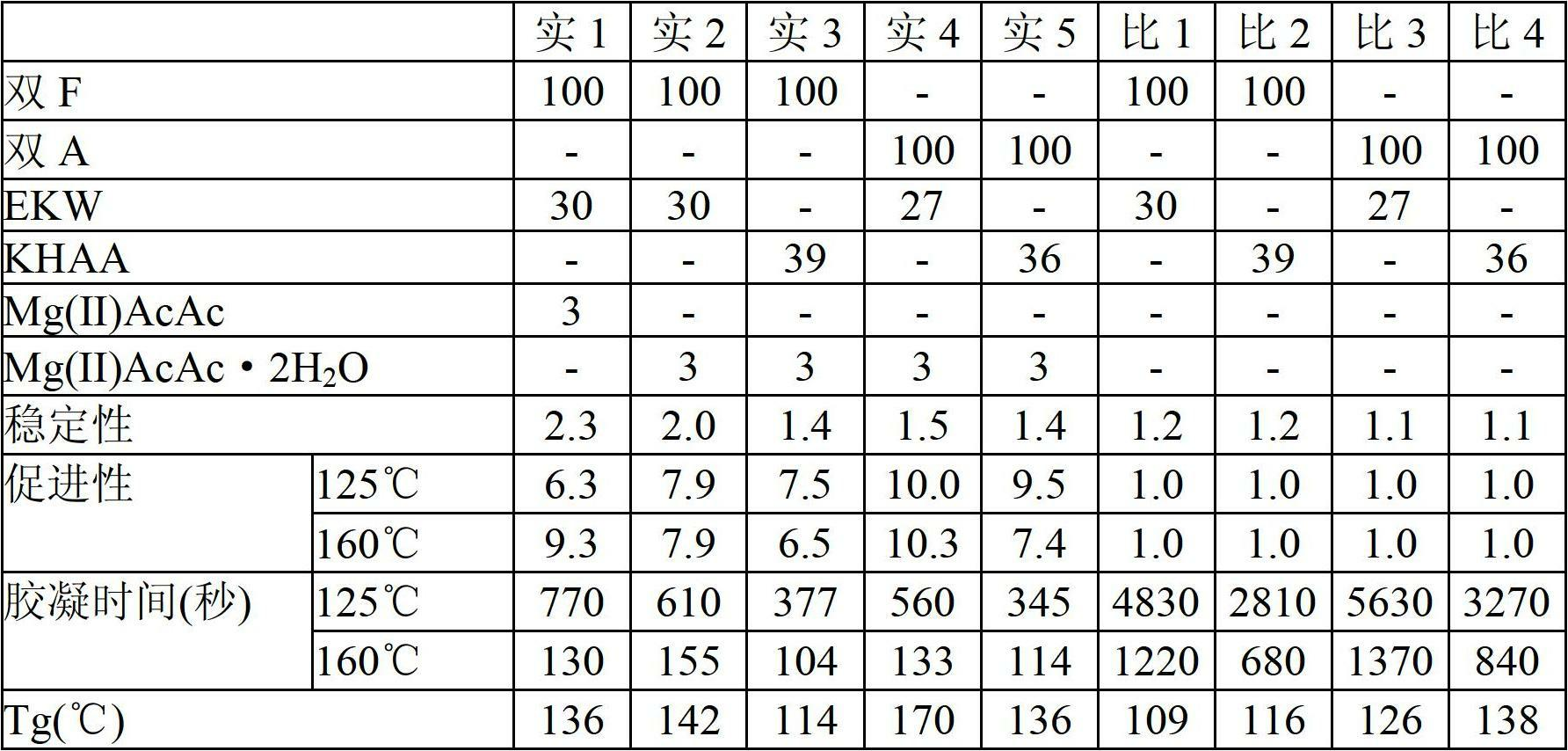
[0066] The purpose of embodiments 1 to 5 and comparative examples 1 to 4 is to use two kinds of epoxy compounds (bisphenol F (recorded as double F in the table) and bisphenol A (recorded as double A in the table)) and two kinds of aromatic Combination of amine curing agent (diethyldiaminotoluene (recorded as EKW in the table) and bis(4-amino-3-ethylphenyl) methane) (recorded as KHAA in the table)), verify magnesium acetylacetonate ( II) (Compounds in which R1 and R2 are methyl groups, R3 is a hydrogen atom, and n is 2 in the above formula (3). The same applies to Examples and Comparative Examples). Comparative Examples 1 to 4 are systems without a curing accelerator, and Example 1 uses 3 parts by weight of magnesium acetylacetonate (II) (recorded as Mg(II)AcAc in the table), and Examples 2 to 5 use 3 parts by weight of acetylacetonate Magnesium (II) acetone hydrate (recorded as Mg(II)AcAc·2H in the table 2 O). The compositions of Examples 1-5 and Comparative Examples 1-4 are...
Embodiment 6~12
[0076] For the epoxy resin composition obtained by changing the addition amount of magnesium acetylacetonate (II) of 100 parts by weight of EXA-830LVP (described as double F in the table), 30 parts by weight of EKW, and curing accelerator , The stability, acceleration and the Tg measurement results of the cured product after curing at 160°C for 90 minutes are listed in Table 2. The evaluation method is the same as that of Examples 1-5.
[0077] [Table 2]
[0078]
[0079] From the results in Table 2, even if magnesium (II) acetylacetonate was added in a small amount of 0.1 parts by weight, the curing acceleration effect was observed, and stability was also observed when it was added up to 15 parts by weight. The preferred addition amount is considered to be between 0.4 and 10 parts by weight.
Embodiment 13~15
[0081] As a curing accelerator, manganese (III) acetylacetonate (represented as Mn(AcAc)3 in the table), manganese (II) acetylacetonate ( It is described as Mn(AcAc)2 in the table), and cobalt(III) acetylacetonate (described as Co(AcAc)3 in the table). Stability, acceleration and Tg of the epoxy resin composition which consists of 100 weight part of EXA-830LVP (it describes as double F in a table|surface) and 30 weight part of EKW were measured similarly to Examples 1-5. The results are listed in Table 3.
[0082] [table 3]
[0083]
[0084] As shown in the results in Table 3, magnesium (II) acetylacetonate can be used as the main curing accelerator and other metal acetylacetonates can be used in combination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com