Ginkgo dipyridamole composition and preparation method of preparation thereof
A damocomposition and the technology of the composition are applied in the direction of drug combination, medical raw materials derived from Ginkgo biloba, and pharmaceutical formulations, etc., which can solve the problems of no control ratio, large toxic and side effects, and low content of active ingredients, and achieve stability and Improved safety, convenient clinical use, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Embodiment 1: the preparation method of ginkgo damocomposition
[0038] Put 10 kg of ginkgo leaf thick shreds into an extraction tank, soak them in 70-80% ethanol for 1-2 hours with 10 times the amount and 8 times the amount, then heat and reflux for extraction for 2 hours and 1 hour respectively, and combine the extraction Liquid, filtered, ethanol was recovered from the filtrate, and an appropriate amount of purified water was added, and filtered with filter paper after standing for 12 hours in refrigeration. Washing with ethanol, collecting eluate of 60% ethanol, recovering ethanol from the eluent until it has no alcohol smell, and concentrating, drying, pulverizing and sieving the collected ointment.
[0039] A certain proportion of dipyridamole is added to the Ginkgo biloba extract prepared by the above method.
[0040] After testing, the percentage by weight of each component in the Ginkgo-Damole composition prepared by the present embodiment is as follows: total ...
Embodiment 2
[0041] Embodiment 2: the preparation method of ginkgo damole composition injection
[0042] Take an appropriate amount of the Ginkgo Damole composition of Example 1, put it into a blender, add water for injection, stir to dissolve, add water for injection to dilute to the configured amount, adjust the pH value to 5.0 with 10% sodium citrate solution, filter, pour Pack into ampoules and sterilize to obtain the ginkgo damole composition injection.
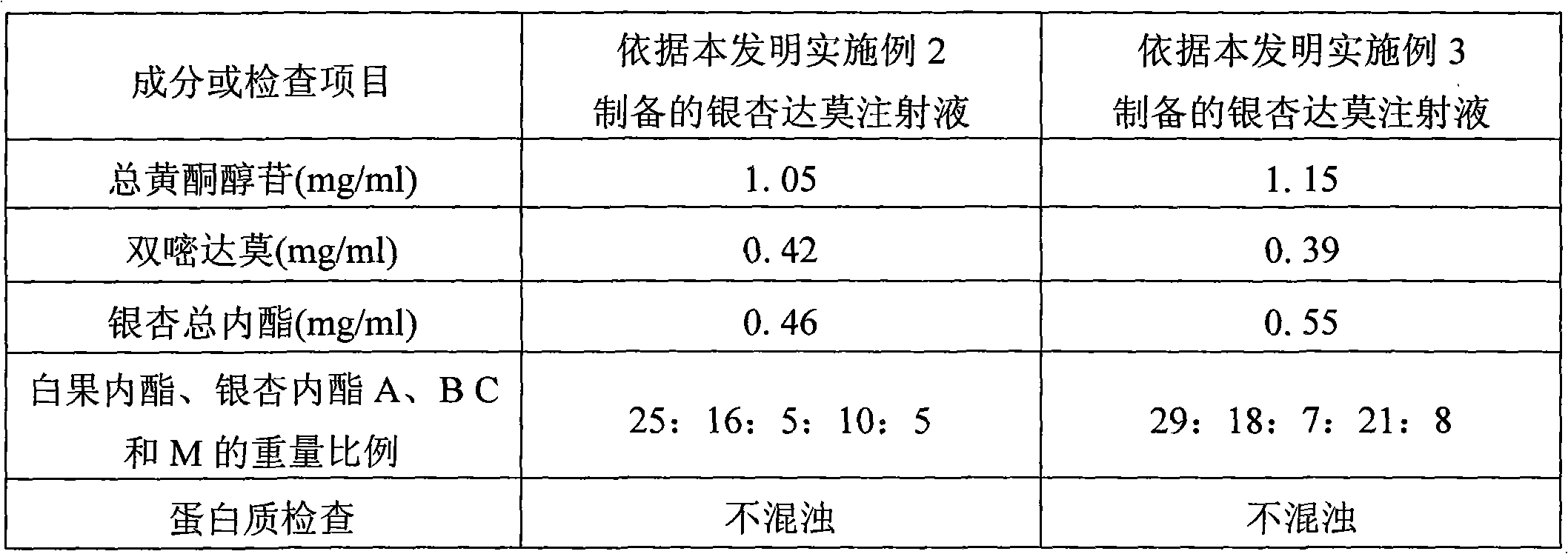
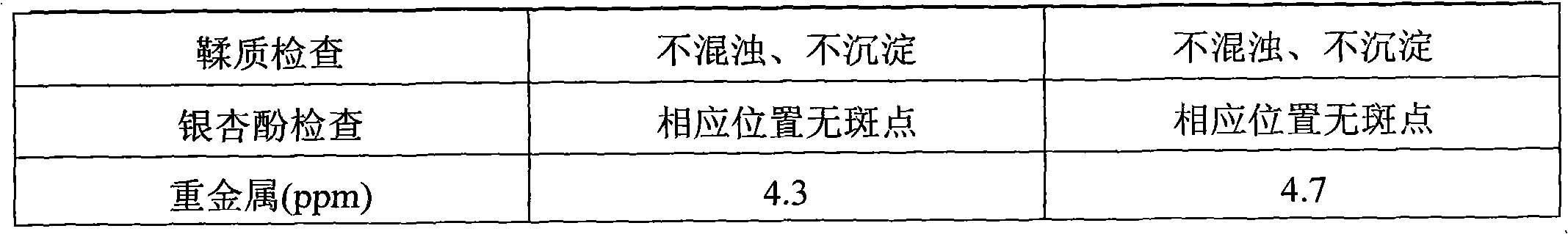
[0043] See Table 2 for the detection results of the contents of each component of the injection prepared in this example.
Embodiment 3
[0044] The preparation of embodiment 3 ginkgo damole composition injection
[0045] Take an appropriate amount of the Ginkgo Damole composition of Example 1, put it into a blender, add water for injection, stir to dissolve, add water for injection to dilute to the configured amount, adjust the pH value to 5.0 with 10% sodium citrate solution, filter, pour Pack into ampoules and sterilize to obtain the ginkgo damole composition injection.
[0046] See Table 2 for the detection results of the contents of each component of the injection prepared in this example.
[0047] The detection result of each component content of injection solution of the present invention of table 2
[0048]
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com