Engineered Escherichia coli and method for producing chondroitin
A technology of Escherichia coli and engineering bacteria, applied in the fields of fermentation engineering and genetic engineering, can solve the problems of low chondroitin yield, yield and production intensity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
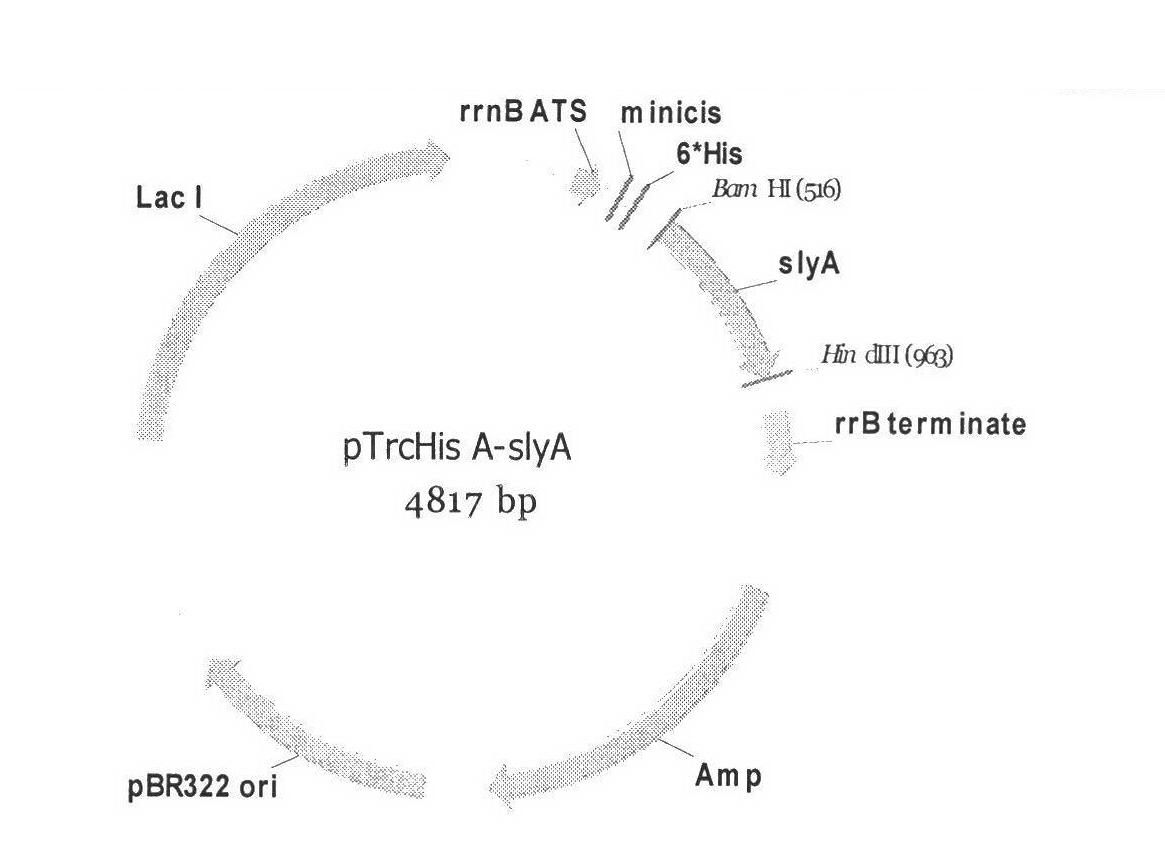
[0022] Embodiment 1: Construction of SlyA expression vector
[0023] Using the Escherichia coli K4 (ATCC 20502) genome as a template, perform PCR according to the method described in the Molecular Cloning Experiment Guide (Third Edition), and then insert the obtained PCR product into pTrcHisA (histidine fusion protein expression vector, Invitrogen) , thus generating pTrcHisA-SlyA.
[0024] Amplification primers:
[0025] SlyAF: 5′-cccaagctttcaccctttggcctgtaactcaat-3′ SlyAR: 5′-cgggatccatgaaattggaatcgccactaggtt-3′
Embodiment 2
[0026] Embodiment 2: SlyA expression vector is expressed in Escherichia coli K4
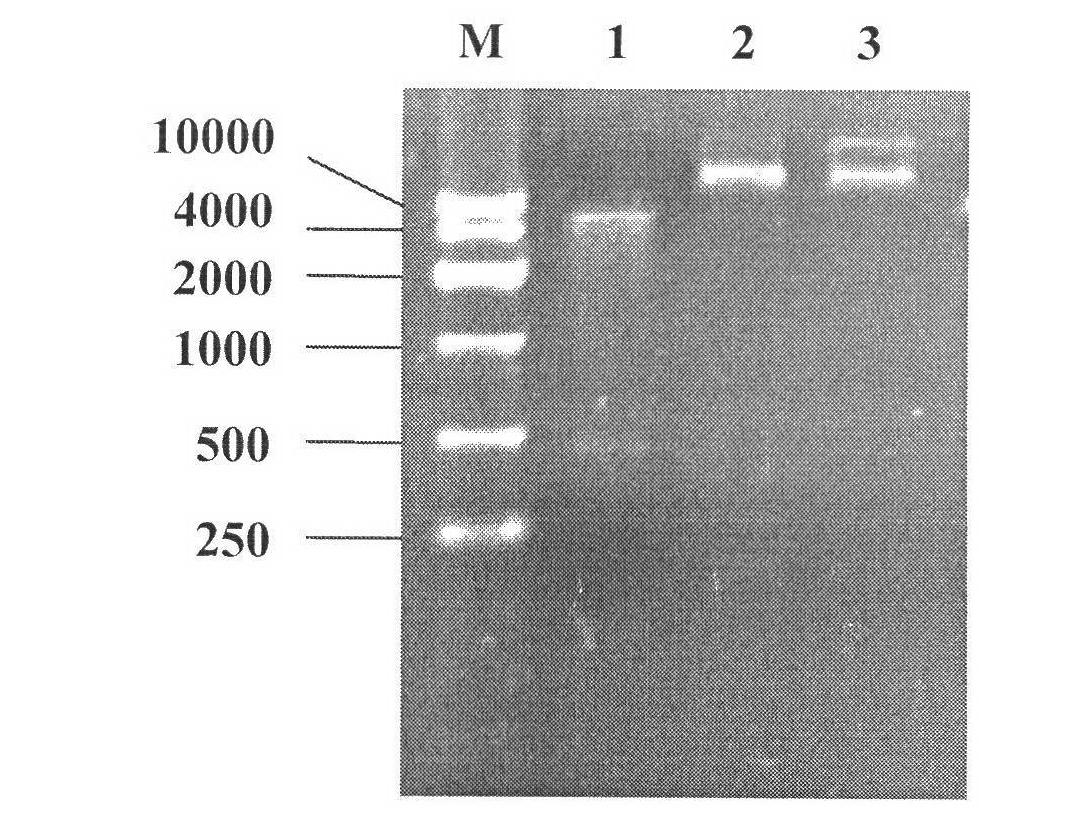
[0027] The expression vector pTrcHisA-SlyA was introduced into Escherichia coli K4 by electroporation transformation method (cell 50 μL, expression vector 2 μL, 200Ω, 25F, 1.8kV, electric shock cup 0.1cm), thereby producing E. coli engineering bacteria. The clones were transferred to LB medium (containing 10 g / L peptone, 10 g / L sodium chloride and 5 g / L yeast powder, natural pH) containing 100 μg / mL ampicillin, and cultured overnight at 37°C. Transfer 1 mL of the culture to 50 mL of fresh LB medium containing 100 μg / mL ampicillin. Incubate at 37°C for 1-3 hours (OD 600 = 0.5-0.8), IPTG was added at a final concentration of 0.5-1.0 mM. Continue culturing for 3-8 hours to induce the expression of recombinant SlyA protein. The cells were collected by centrifugation of 1 mL of induction culture medium, followed by SDS-PAGE electrophoresis according to the Molecular Cloning Experiment Guide (Third ...
Embodiment 3
[0028] Example 3: Production of capsular polysaccharide by fermentation
[0029] Inoculate the Escherichia coli engineered bacteria in LB medium containing 100 μg / mL ampicillin, and perform seed culture at 37° C. for 10-14 hours. Then transfer to 100μg / mL GY medium (containing 10g / L glycerol, 1g / L peptone, 1g / L ammonium chloride, 0.5g / L trisodium citrate, 0.1g / L oxidized Magnesium, 9.7g / L dipotassium hydrogen phosphate and 2g / L potassium dihydrogen phosphate, pH 7.2~7.4), cultured at 37°C for 1-3 hours (OD 600 =0.5-0.8), add IPTG with a final concentration of 1 mM, and continue culturing for 24 hours. During the whole culture process, Escherichia coli K4 was used as the control, but no ampicillin was added.
[0030] The content of capsular polysaccharide in the fermentation broth was detected according to the carbazole method described in Microbial Cell Factories, 2010, 9: 34-43. Table 1 is the comparison of the fermentation results of Escherichia coli engineering bacteria ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com