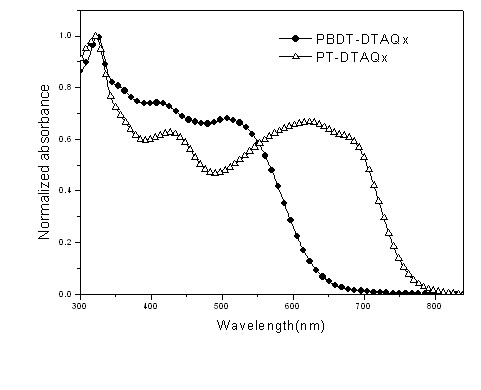
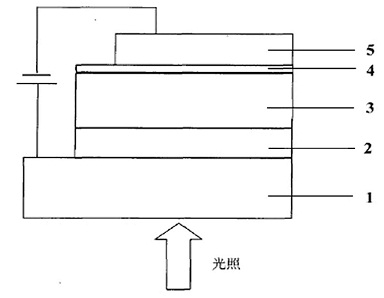
Conjugated polymer of polyacenaphthylene and quinoxaline, as well as preparation method and application of conjugated polymer
A technology of conjugated polymers and quinoxaline, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of unsatisfactory photovoltaic performance and low open circuit voltage of devices, so as to improve photovoltaic performance, Effects of increasing molecular weight and improving morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 polymer PBDT-DTAQx
[0031] (1) Preparation of 5,8-bis(2-bromo-3-substituted thienyl)-5-acenaphthoquinoxaline monomer
[0032]
[0033] Referring to the synthesis method disclosed in Angewandte Chemie International Edition 2009, 48, 1664-1668, benzothiadiazole was prepared by reacting o-phenylenediamine with thionyl chloride as a starting material, and 4,7- Dibromobenzothiadiazole, then reduced by sodium borohydride to give 3,6-dibromo-o-phenylenediamine. Condensation of 3,6-dibromo-o-phenylenediamine and acenaphthoquinone to obtain 5,8-dibromoacenaphthoquinoxaline, and finally through Suzuki Coupling and bromination to obtain polymerized monomer 5,8-bis(2-bromo -3-dodecyl-5-thienyl)-acenaphthoquinoxaline.
[0034] The following takes the preparation of 5,8-bis(2-bromo-3-dodecyl-5-thienyl)-acenaphthoquinoxaline as an example to describe in detail, and the synthesis steps are as follows:
[0035] (1) Preparation of benzothiadiaz...
Embodiment 2
[0059] The preparation of embodiment 2 polymer PT-DTAQx
[0060]
[0061] 5,8-bis(2-bromo-3-dodecyl 5-thienyl)-acenaphthoquinoxaline (725 mg, 0.79 mmol), 2,5-bis(tributyltin)-thiophene (526 mg , 0.79 mmol), tetrakistriphenylphosphine palladium (45 mg) was added to the reaction flask, replaced with high-purity argon three times, and then injected with anhydrous and oxygen-free toluene, under the protection of argon, heated and refluxed for 72 h, the reaction After completion, the obtained polymer was settled in methanol, filtered with suction, and the collected polymer was subjected to Soxhlet extraction with acetone and n-hexane in turn for 24 h, and vacuum-dried at 40 °C for 24 h to obtain 0.42 g of a black solid, with a yield of 62%. . 1 H NMR (400 MHz, CDCl 3 , ppm) : 8.46 (br, 2H), 8.11 (br, 4H), 7.86~7.66 (br, 4H), 7.57~7.52 (br, 2H), 2.87~2.73 (br, 4H), 1.56~1.25 (br , 40H), 0.87 (br, 6H).
Embodiment 3
[0062] The preparation of embodiment 3 polymer PC-DTAQx
[0063]
[0064] Under argon protection, 5,8-bis(2-bromo-3-dodecyl 5-thienyl)-acenaphthoquinoxaline (343 mg, 0.38 mmol), N-(2-ethylhexyl )-3,6-carbazole dipinacol ester (200 mg, 0.38mmol), Pd(PPh 3 ) 4 (22 mg), 10 mL of toluene and 10 ml of 2 M potassium carbonate solution were added to a two-neck flask, heated to 90-95 °C under dark conditions, and stirred for 72 h. After the reaction, it was naturally cooled to room temperature, extracted with chloroform, washed with saturated brine, and dried overnight with anhydrous magnesium sulfate. Filter off the desiccant, spin off the solvent, settle with a large amount of methanol, and filter with suction. The collected polymer is subjected to Soxhlet extraction with acetone and n-hexane in turn for 24 h, and vacuum-dried at 40 °C for 24 h to obtain 0.22 g of a black solid. Yield 52%. 1 H NMR (400 MHz, CDCl 3, ppm): 8.36~8.33 (br,2H), 8.07~8.05 (br,4H), 7.96~7.70 (br,8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Open circuit voltage | aaaaa | aaaaa |
| Short circuit current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com