Process for preparing alpha-acetyl-gamma-butyrolactone for co-production of various phosphates
A technology of butyrolactone and acetyl, which is applied in the field of co-production of various phosphates while preparing α-acetyl-γ-butyrolactone, so as to avoid environmental pollution, reduce production cost and improve the conditions of comprehensive utilization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Preparation of α-acetyl-γ-butyrolactone: Add 11.70g γ-butyrolactone, 17.90g ethyl acetate Ester and catalyst metal sodium 3.43g, carry out acylation, then lower the temperature, when the temperature drops to 62°C, add 26.95g of 51% phosphoric acid solution dropwise under stirring, after the addition, fully stir, let stand to separate layers, The aqueous phase was separated over time. The organic phase was rectified (vacuum degree: 0.08Mp) to obtain 15.90 g of α-acetyl-γ-butyrolactone with a purity of 99.1%.
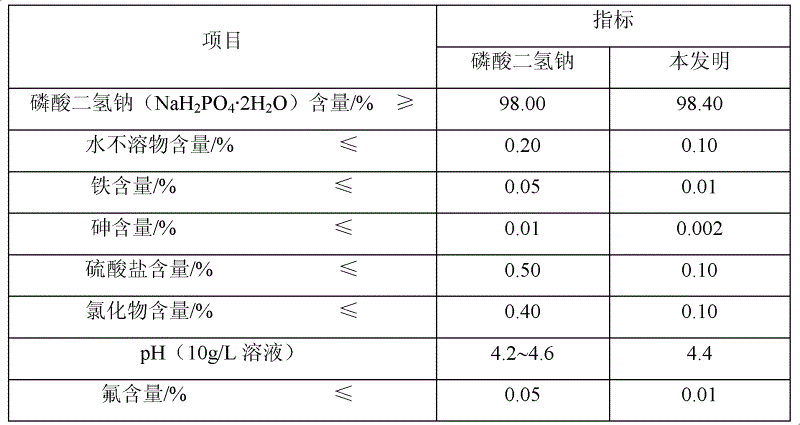
[0019] 2) Preparation of sodium dihydrogen phosphate: adjust the pH value of the water phase to 3.0 with 51% phosphoric acid, concentrate, cool down to room temperature, filter under reduced pressure to obtain 2110 g of sodium dihydrogen phosphate dihydrate, and continue to dehydrate at a constant temperature of 120 ° C for 30 minutes to obtain no Sodium dihydrogen phosphate water 16.23g, product analysis index and industrial sodium dihydrogen phosphate standar...
Embodiment 2
[0023] 1) Preparation of α-acetyl-γ-butyrolactone: the oil-water separation temperature was 61° C., and the rest were the same as in Example 1 to obtain 15.66 g of α-acetyl-γ-butyrolactone with a purity of 99.3%.
[0024] 2) Preparation of sodium hexametaphosphate: the aqueous phase was adjusted to pH 2.8 with 51% phosphoric acid, concentrated, cooled to room temperature, filtered under reduced pressure to obtain 20.60 g of sodium dihydrogen phosphate dihydrate, and dehydrated at 120° C. for 30 minutes to obtain no Sodium dihydrogen phosphate in water, then polymerize it at a constant temperature at 700°C for 30 minutes, then pour the molten liquid into an iron container cooled by a water bath, cool to room temperature, crush it with a pulverizer, and pass through a 35-mesh sieve , to get 12.61g sodium hexametaphosphate. The product analysis results and the indicators of sodium hexametaphosphate standard HG / T2519-2007 are listed in Table 2. The sodium hexametaphosphate prepar...
Embodiment 3
[0029] 1) The preparation of α-acetyl-γ-butyrolactone is the same as in Example 1.
[0030] 2) Preparation of sodium trimetaphosphate: adjust the pH value of the water phase to 3.0 with 51% phosphoric acid, concentrate, cool down to room temperature, filter under reduced pressure to obtain 2110 g of sodium dihydrogen phosphate dihydrate, and continue to dehydrate at 120°C for 30 minutes to obtain anhydrous 16.23 g of sodium dihydrogen phosphate, then polymerized at a constant temperature of 550°C for 5 hours, then cooled to room temperature, crushed with a pulverizer, and passed through a 35-mesh sieve to obtain 12.70 g of sodium trimetaphosphate. The content determination of sodium trimetaphosphate is carried out by the method of "research on the content determination method of industrial grade sodium trimetaphosphate", and the index of the product is shown in Table 3.
[0031] The sodium trimetaphosphate index produced by the present invention of table 3
[0032] p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com