Preparation method and medicinal application of ginsenoside Rh2 aliphatic ester compound
A technology of ginsenosides and fatty acid esters, applied in steroids, pharmaceutical formulations, organic chemistry, etc., can solve the problems of no artificial synthesis products reported in literature, and achieve the effect of less impurities, high yield and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0050] a) Ginsenoside secondary glycoside Rh 2 , the sample was purified by conventional silica gel column chromatography and recrystallization to obtain pure product.
[0051] b) take Rh 2 5g was dissolved in 100mL chloroform and set aside. Measure octanoyl chloride 4.1g, triethylamine 4.5g, put in 100mL chloroform solution, react for 15-30min, then slowly add Rh 2 chloroform solution. After 12-48 hours of reaction, chloroform was recovered under reduced pressure to obtain dry matter.
[0052] c) The dry matter was dissolved in methanol, filtered, ODS column chromatography, eluting with methanol and water (80%-100%), and the combined target product was detected by thin-layer chromatography, and recovered under reduced pressure to obtain 3.27 g of dry matter. That is, ginsenoside secondary glycoside fatty acid ester derivatives, wherein the fatty acyl group is C 8 The fatty acyl group (the fatty acyl group is connected at the C-12 position of the mother nucleus).
Embodiment 3
[0054] a) Ginsenoside secondary glycoside Rh 2 , the sample was purified by conventional silica gel column chromatography and recrystallization to obtain pure product.
[0055] b) take Rh 2 3.0g was dissolved in 100mL chloroform and set aside. Measure 3.2g of fatty acid chloride and 3.5g of potassium carbonate, place in 100mL chloroform solution, suspend for 15min, and then slowly add Rh 2 chloroform solution. After reacting for 12-24h, chloroform was recovered under reduced pressure to obtain dry matter.
[0056] c) is the same as c) in the example (2).
[0057] 4.4. Embodiment 4
[0058] a) Ginsenoside secondary glycoside Rh 2 , the sample was purified by conventional silica gel column chromatography and recrystallization to obtain pure product.
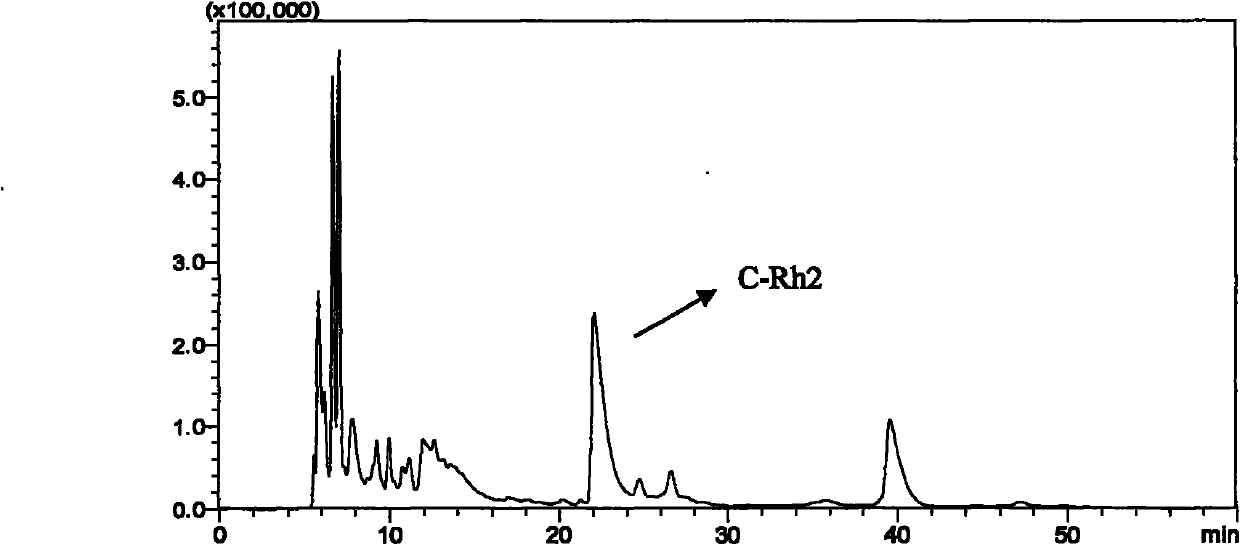
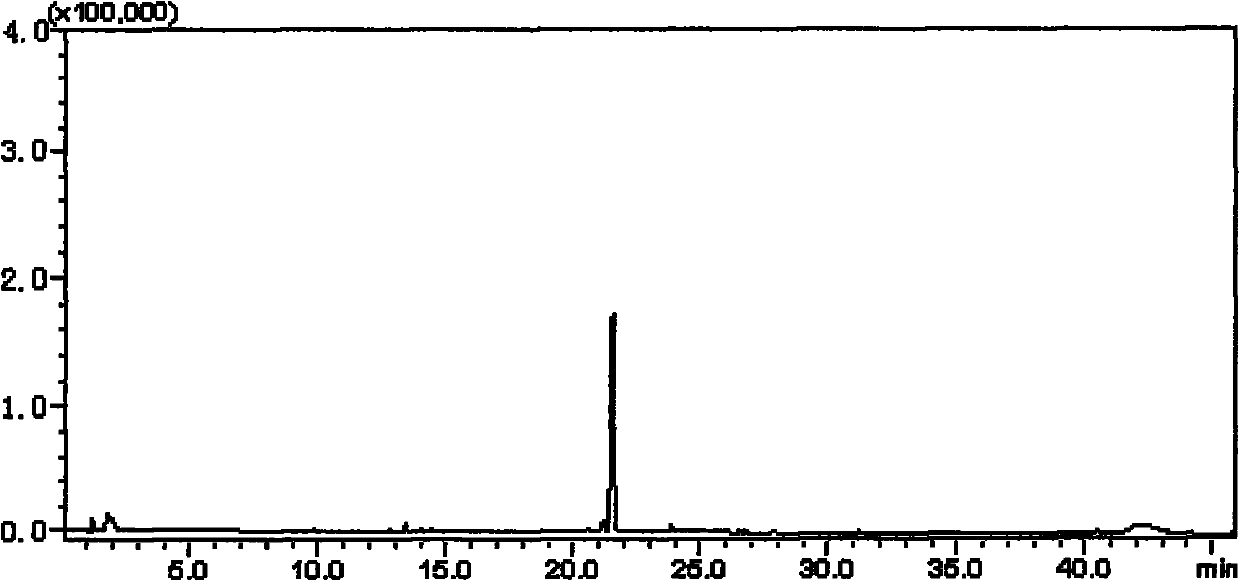
[0059] b) Accurately weigh ginsenoside Rh 2 50.0mg, synthesize C-Rh with the method in example 1 2 , The obtained dry matter was fixed to volume in a 50 ml volumetric flask with 50 ml of chromatographic methanol, and the...
experiment example 7
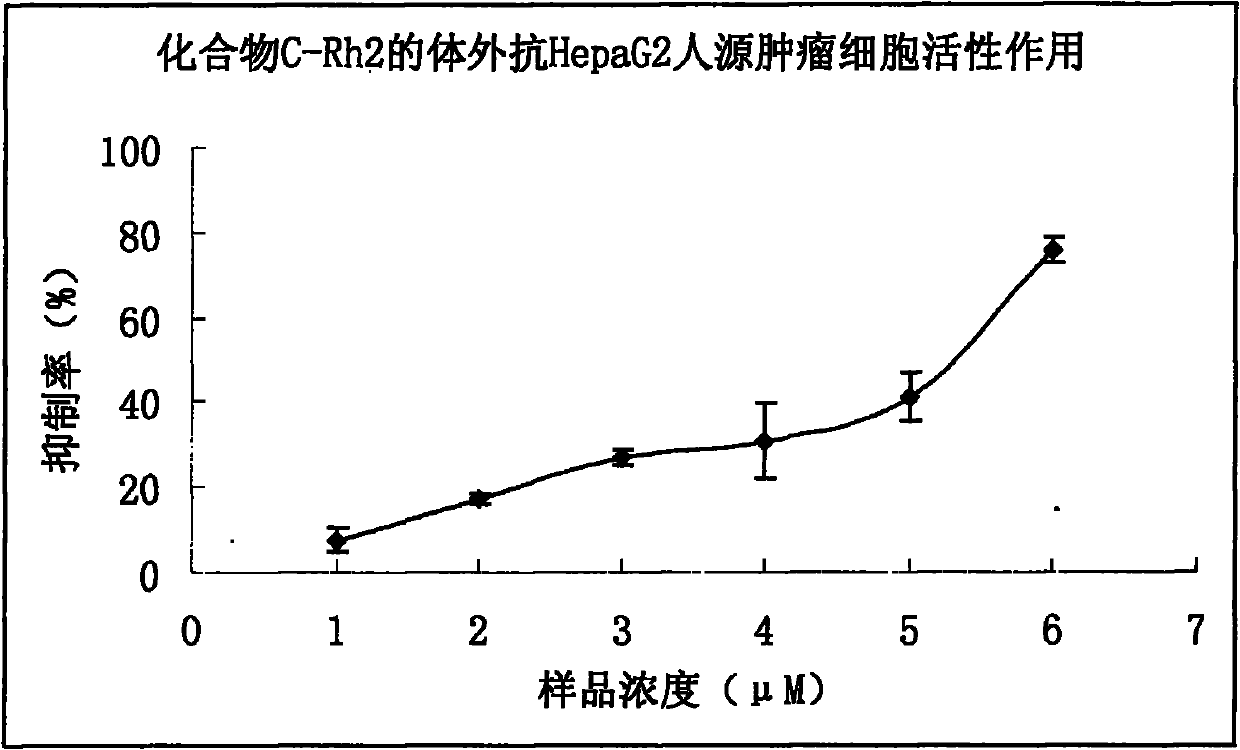
[0091] 4.7 Experimental Example 7 Compound C-Rh 2 Anti-HepaG2 Human Tumor Cell Effect in Vitro
[0092] 4.7.1 Experimental materials and methods
[0093] Experimental materials: Cell lines: HepaG2 human tumor cells are cell lines preserved in the pharmacology laboratory of Bethune School of Medicine, Jilin University. Synthetic product C-Rh 2 .
[0094] method:
[0095] 1. HepaG2 cells were revived, cultured in 1640 medium containing 10% fetal bovine serum for 24 hours, and then cultured in serum-free 1640 medium for 24 hours.
[0096] 2. Select the HepaG2 cells treated above (in the logarithmic growth phase) and inoculate them in a 96-well culture plate. The experimental group is replaced with a new medium containing the samples to be tested at different concentrations, and the control group is replaced with a medium containing an equal volume of solvent. , set 3 parallel wells for each group, and set up a control well for the drug solution, at 37°C in 5% CO 2 Culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com