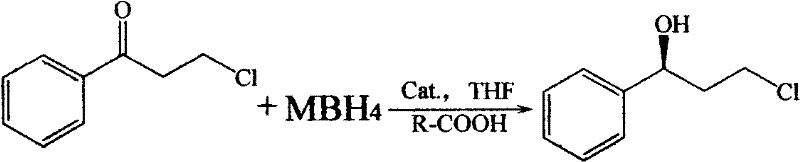Method for asymmetrically catalyzing and synthesizing (R)-(+)-3-chlorine-phenylpropanol
An asymmetric, phenylpropanol technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of difficulty in the source and storage of reactants, increase the reaction cost, and the product yield is less than 50%, and achieves high yield and product optical properties. Purity, reduced production and storage costs, improved catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
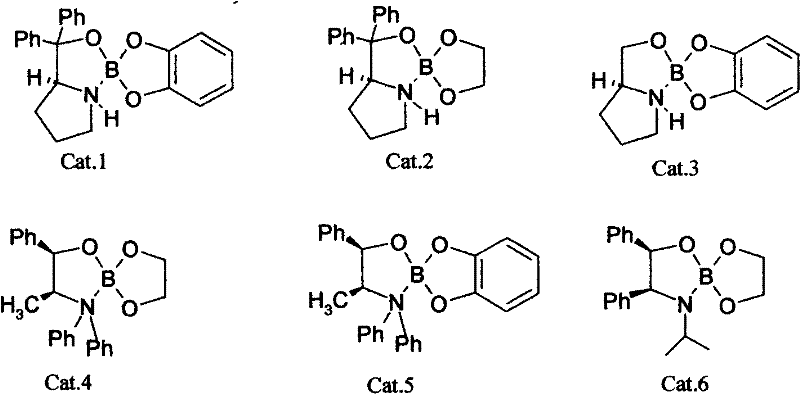
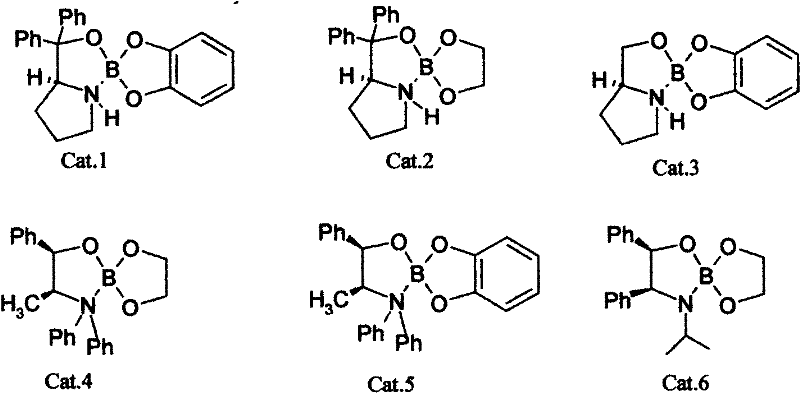
[0026] Weigh catalyst Cat.2, 0.544g (1.75nmol) and KBH 4 Put 0.55g (12.5mmol) in a 100ml three-necked flask, add 15ml tetrahydrofuran solution and mix, add glacial acetic acid dropwise to the flask until the pH value of the reaction solution is 1.0, stir the reaction to turn the mixed solution into a clear and transparent liquid. Put 25ml of 3-chloro-propiophenone tetrahydrofuran solution (0.8mol / L) into the constant pressure funnel, slowly drop it into the reaction bottle at a rate of 2S / drop, and drop it for about 0.5h. After 4h of reaction, add ice water to terminate the reaction . The resulting solution was analyzed by chiral column gas chromatography to obtain a conversion rate of 92% and an optical purity e.e. value of 96%.
[0027] The reaction solution was washed with 2.0mol / L hydrochloric acid solution, the organic layer was left to pass through the column, and the effective eluent was decompressed and rotary evaporated to remove 3.0 g of off-white solid (R)-(+)-3-c...
Embodiment 2
[0030] Weigh catalyst Cat.6, 0.81g (2.5nmol) and NaBH 4 , 0.95g (25mmol) in a 100ml three-necked flask, add 15ml tetrahydrofuran solution and mix, add glacial acetic acid dropwise to the bottle until the pH value of the reaction solution is 5.0, stir the reaction to make the mixed solution become a clear and transparent liquid. Put 20ml of 3-chloro-propiophenone tetrahydrofuran solution (1.25mol / L) into the constant pressure funnel, and slowly drop it into the reaction bottle at a rate of 2S / drop. The dropwise addition is completed in about 0.5h. After 5h of reaction, add ice water to terminate the reaction. The resulting solution was analyzed by chiral column gas chromatography to obtain a conversion rate of 95% and an optical purity e.e. value of 94%.
[0031] The reaction solution was washed with 2.0 mol / L hydrochloric acid solution, and the organic layer was left for rotary evaporation under reduced pressure, and the obtained crude product was recrystallized with cyclohexa...
Embodiment 3
[0034] Weigh catalyst Cat.3 (see chemical formula) 1.6275g (7.5nmol) and LiBH 4 1.1g (50mmol) in a 100ml three-necked flask, add 15ml tetrahydrofuran solution and mix, add glacial acetic acid dropwise to the bottle until the pH of the reaction solution is 0.2, stir the reaction to make the mixed solution become a clear and transparent liquid. Put 18ml of tetrahydrofuran solution (1.5mol / L) of 3-chloro-propiophenone into the constant pressure funnel, and slowly drop it into the reaction bottle at a rate of 2S / drop. After about 0.5h, the dropwise addition is completed. After 2h of reaction, add ice water to terminate the reaction . The resulting solution was analyzed by chiral column gas chromatography to obtain a conversion rate of 98% and an optical purity e.e. value of 93%.
[0035] The reaction solution was washed with 2.0 mol / L hydrochloric acid solution, the organic layer was left for decolorization, and the crude product obtained by rotary evaporation under reduced pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com