Dropping pills for preventing and curing hypertension and hyperlipidemia and preparation method
A technology for hyperlipidemia and high blood pressure, which is applied in the field of dripping pills for the prevention and treatment of hypertension and hyperlipidemia and its preparation. It can solve the problems of low dissolution rate and bioavailability, complex production process, inconvenient taking, etc., and it is easy to meet the production conditions. Effects of control, high bioavailability, ease of use and portability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
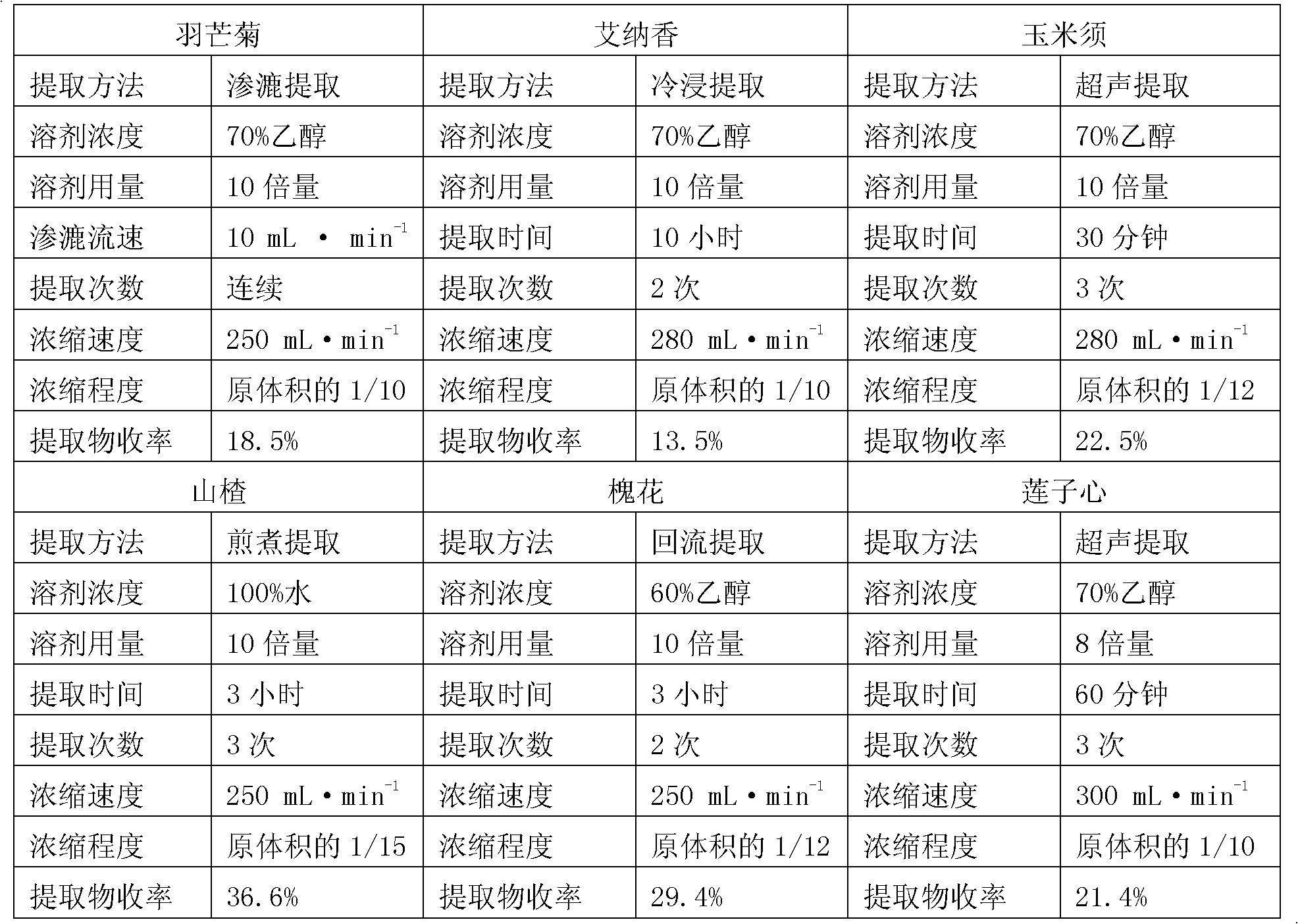
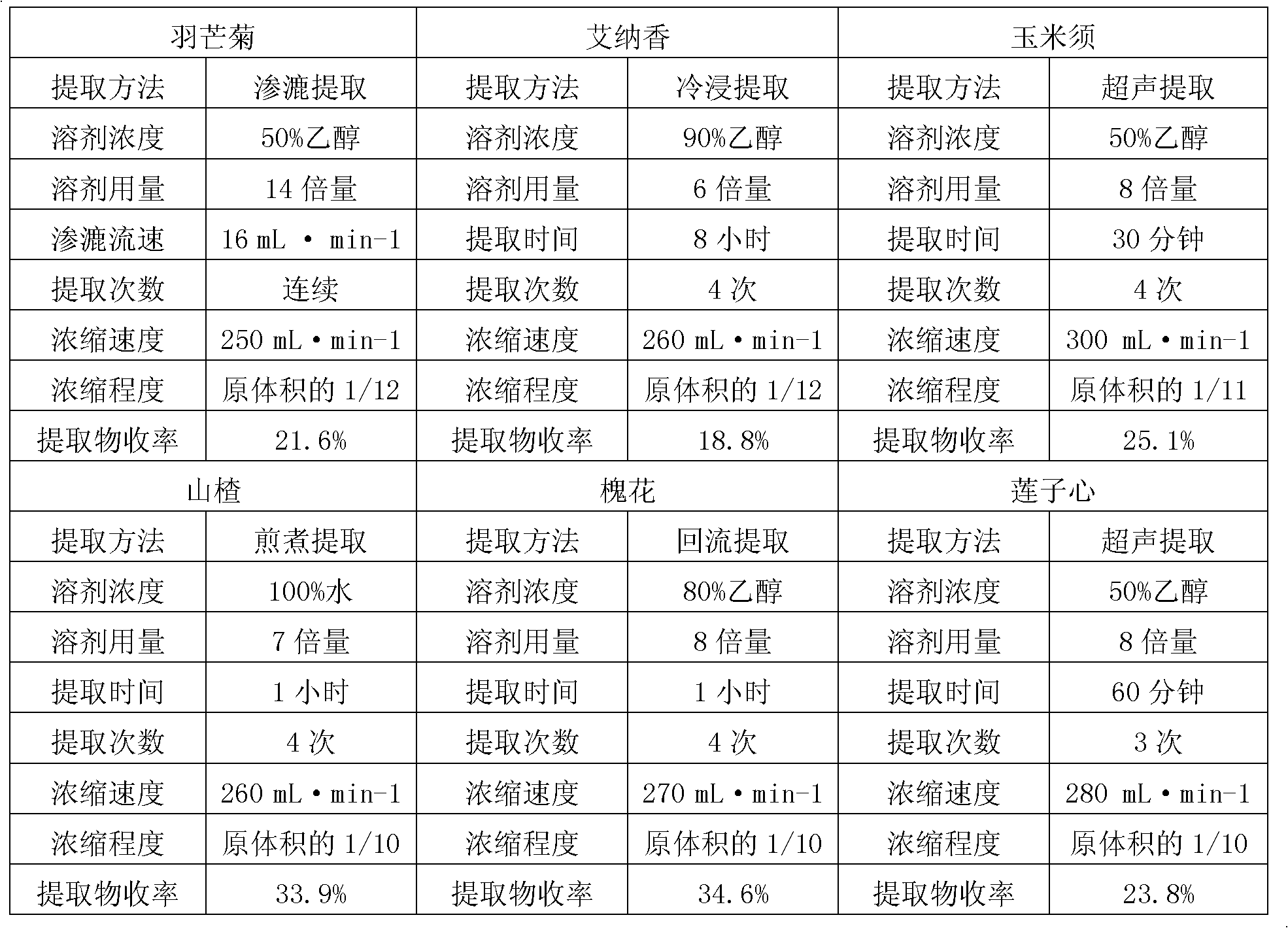
[0024]The dropping pills of the present invention are made up of the following weight ratios: 8-25% of the extract of Pinegrass chrysanthemum, 10-23% of the extract of Ainaxiang, 15-25% of the extract of corn silk, 12-25% of the extract of hawthorn, 12-25% of the extract of Sophora japonica The total amount of 10-20% of the extract and 8-15% of the lotus seed extract is 100% as the raw material drug and the mixed matrix, and the weight ratio of the raw material drug to the mixed matrix is 1:3-5, wherein the mixed matrix is heated Melt; add the raw drug into the melted mixed matrix, stir evenly at 80°C, keep warm at 75-80°C for 20-30 minutes to discharge the bubbles, and obtain a molten liquid; adjust the dripper temperature of the dropping pill machine to 75- 80°C, use one of liquid paraffin, soybean oil or simethicone as the condensate, keep the temperature of the condensate at 6-10°C, put the molten liquid in the liquid storage tank of the dropping pill machine at a drop ...
Embodiment 2
[0026] The dropping pills of the present invention are composed of the following weight ratios: 10-22% of the extract of Featherflower, 15-20% of the extract of Ainaxiang, 15-22% of the extract of corn silk, 15-23% of the extract of hawthorn, and 15-23% of the extract of Sophora japonica. The total amount of 12-18% of the extract and 10-14% of the lotus seed extract is 100% as the raw drug and the mixed matrix, and the weight ratio of the raw drug to the mixed matrix is 1:3-5, wherein the mixed matrix is heated Melt; add the raw drug into the melted mixed matrix, stir evenly at 80°C, keep warm at 75-80°C for 20-30 minutes to discharge the bubbles, and obtain a molten liquid; adjust the dripper temperature of the dropping pill machine to 75- 80°C, use one of liquid paraffin, soybean oil or simethicone as the condensate, keep the temperature of the condensate at 6-10°C, put the molten liquid in the liquid storage tank of the dropping pill machine at a drop distance of 5-10°C....
Embodiment 3
[0028] The dropping pills of the present invention are made of the following weight ratios: 20% of Featherflower extract, 20% of Ainaxiang extract, 20% of corn silk extract, 20% of Hawthorn extract, 10% of Sophora japonica flower extract, and 10% of lotus seed heart extract The total amount of 10% of the product is 100% as raw drug and mixed matrix, and the weight ratio of raw drug and mixed matrix is 1:3, wherein, the mixed matrix is heated and melted; the raw drug is added to the melted mixed matrix, Stir evenly at 80°C, keep warm at 75-80°C for 20-30 minutes to discharge air bubbles, and obtain a molten liquid; adjust the dripper temperature of the dropping pill machine to 75-80°C, use liquid paraffin as the condensate, and the temperature of the condensate Keep it at 6-10°C, put the molten liquid in the liquid storage tank of the dropping pill machine, drop it into the condensate under the conditions of a drop distance of 5-6 cm and a drop speed of 20-60 drops / minute, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com