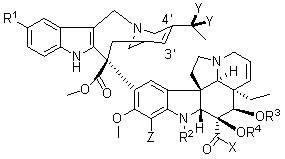
Vinblastine derivative, preparation method of vinblastine derivative, and application of vinblastine derivative in medicines
A technology of compounds, general formulas, applied in the field of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
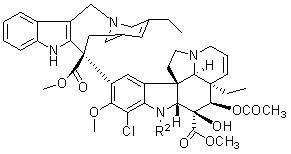
[0041] Example 1 Preparation of 17'-chlorovinorelbine
[0042]
[0043]
[0044] Under argon atmosphere, vinorelbine (0.387 g, 0.5 mmol) and ferrocene chloride [Cp 2 TiCl 2 , 25 mg, 0.1 mmol] dissolved in 10-80 ml of dichloromethane solvent under stirring, at ambient temperature, add chlorosuccinimide (0.2 g, 1.5 mmol) in batches or at one time, continue stirring, point After following the reaction, the raw materials disappeared. The reaction solution was poured into saturated sodium bisulfite solution (50 ml), the pH of the solution was adjusted to 8 with ammonia water, and the reaction solution was extracted with ethyl acetate (100 ml×3). The organic phases were combined, washed successively with saturated sodium bicarbonate solution (50 ml) and saturated sodium chloride solution (50 ml), the ethyl acetate layer was dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure, and purified by silica gel column chromatography. ...
Embodiment 2
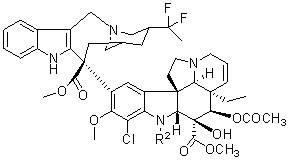
[0046] Embodiment 2: Preparation of 17'-chloro vinflunine
[0047]
[0048]
[0049] Under argon atmosphere, in a dry three-necked flask, vinflunine (0.7 g, 0.857 mmol) and ferrocene chloride [Cp 2 TiCl 2 , 21 mg, 0.0857 mmol] dissolved in 10-80 ml of dichloromethane solvent under stirring, at ambient temperature, add chlorosuccinimide (0.458 g, 3.43 mmol) in batches or at one time, continue stirring, point After following the reaction, the raw materials disappeared. The reaction solution was poured into saturated sodium bisulfite solution (50 ml), the pH of the solution was adjusted to 8 with ammonia water, and the reaction solution was extracted with ethyl acetate (100 ml×3). The organic phases were combined, washed successively with saturated sodium bicarbonate solution (50 ml) and saturated sodium chloride solution (50 ml), the ethyl acetate layer was dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure, and purified...
Embodiment 3
[0052] (1) Materials: human lung adenocarcinoma A549 cell line (purchased from Shanghai Institute of Cell Biology); recombinant human tumor necrosis factor (rhTNF-α, referred to as TNF); recombinant human interferon γ (rhIFN-γ, referred to as IFN); RPMI-1640 medium (Japan).
[0053] (2) Method: The whole experiment was set up as a blank zero group (plus 200 μl of medium), a cell control group (100 μl of single cell suspension + 100 μl of medium), an experimental group (100 μl of single cell suspension + 100 μl of each treatment factor ). Using Mossman [1] tetramethyl azolium blue (MTT) colorimetric method. A549 cells were cultured in RPMI-1640 medium containing 15% calf serum at 37°C and 5% CO 2 Cultivate in an incubator, digest with 0.25% trypsin and passage after 2 to 3 days. Adjust the digested and counted single-cell suspension to 4×105 / ml and add it to a 96-well culture plate, 100 μl per well, add 100 μl of drug to the experimental group, set 3 replicate wells in each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com