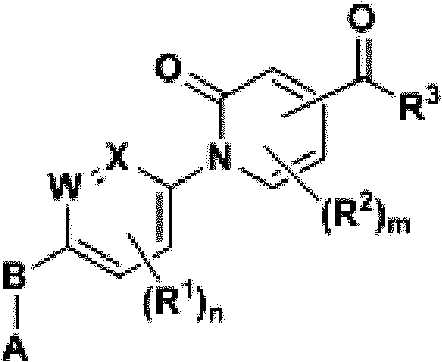
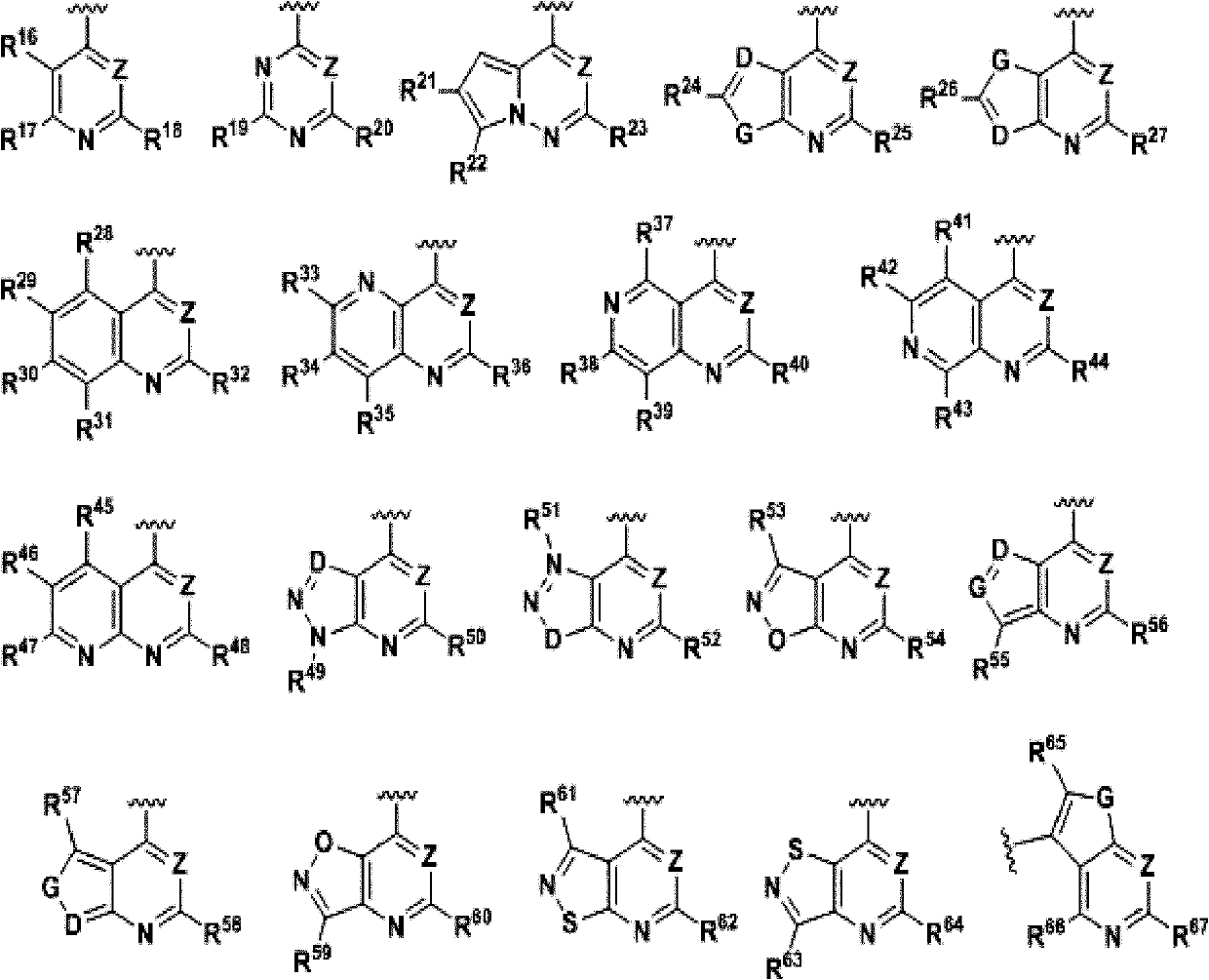
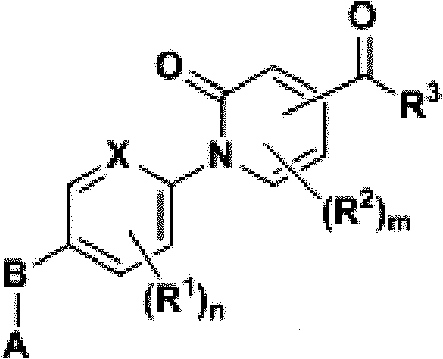
Compound, and preparation method and application thereof
A technology of compounds, equivalents, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0495] Embodiment 1: preparation compound 67
[0496]
[0497] Weigh 1.27g of about 10mmol of compound S1, 1.71g of about 10mmol of benzyl bromide, and 1.38g of about 10mmol of potassium carbonate in a 250mL reaction flask, add 100mL of DMF, stir at room temperature overnight, concentrate to obtain a yellow solid, and use Petroleum ether-ethyl acetate was eluted according to a gradient of 0-25%, and 1.85 g of yellow oily compound S2 was obtained with a yield of 85%.
[0498] Weigh 1.09g of about 5mmol of compound S2, 0.77g of about 5mmol of compound S3, and 0.61g of about 5mmol of DMAP in a 250mL reaction flask, add 100mL of ethanol, stir at 90°C for 2h, concentrate to obtain a yellow solid, and use Petroleum ether-ethyl acetate was eluted according to a gradient of 0% to 45% R, to obtain 1.41 g of yellow solid compound S4 with a yield of 80%.
[0499] Weigh 1.41g of about 4mmol of compound S4, 0.42g of about 4mmol of Pd-C, stir at room temperature in a hydrogen atmosphere...
Embodiment 2
[0505] Embodiment 2: preparation compound 68
[0506]
[0507] Weigh 1.74g of about 10mmol of compound S7 and 1.19g of about 10mmol of DMF-DMA into a 250mL reaction bottle, add 100mL of DMF, stir at 90°C for 2h, concentrate to obtain a yellow solid, and use petroleum ether-ethyl acetate According to the gradient elution of 0-15%, 2.06 g of compound S8 was obtained as a yellow solid with a yield of 90%.
[0508] Weigh 2.06g of about 9mmol of compound S8, 1.95g of about 9mmol of compound S2, and 1.10g of about 9mmol of DMAP, put them in a 250mL reaction bottle, add 100mL of ethanol, stir and react at 90°C for 2h, and concentrate to obtain a yellow solid. Eluting with petroleum ether-ethyl acetate according to a gradient of 0-45%, 2.82 g of yellow solid compound S9 was obtained with a yield of 85%.
[0509] Weigh 1.84g of about 5mmol of compound S9, 1.42g of about 10mmol of iodomethane, and 0.69g of about 5mmol of potassium carbonate in a 250mL reaction bottle, add 100mL of ace...
Embodiment 3
[0516] Embodiment 3: preparation compound 69
[0517]
[0518] Weigh 1.77 about 5 mmol of compound S4, 0.2 g of about 5 mmol of sodium hydroxide, 50 mL of water, stir at 90°C for 5 h, neutralize with 3 mol / L HCl to pH = 7, filter with suction, and dry to obtain 1.53 g of a yellow solid Compound S13, yield 90%.
[0519] Weigh 1.36 about 4 mmol of compound S13, 0.81 mg of about 4 mmol of EDCI, 0.55 g of about 4 mmol of HOBt, 0.2 g of about 1.64 mmol of DMAP, and 0.5 g of about 4 mmol of p-fluorobenzylamine in a 250 mL reaction bottle 50 mL of DMF and 50 mL of dichloromethane were added, stirred overnight at room temperature, and concentrated to obtain a yellow solid, which was eluted with a gradient of 0% to 5% with methanol-dichloromethane to obtain 1.07 g of yellow solid compound S14 with a yield of 75%.
[0520] Weigh 0.89g of about 2mmol of compound S14, 0.21g of about 2mmol of Pd-C, in a hydrogen atmosphere, stir at room temperature overnight, concentrate to obtain a ye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com