Multilayered composite tube, manufacturing method thereof, and application thereof
A tube and inner layer technology, applied in the field of medical devices, can solve the problems of imperfect drug release system, drug shedding, drug loss, etc., to save spraying drugs, improve production efficiency, and shorten the production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Component A: PCL500g Component B: PCL500g
[0051] Drug 1: Cilostazol 10g Drug 2: Rapamycin 10g
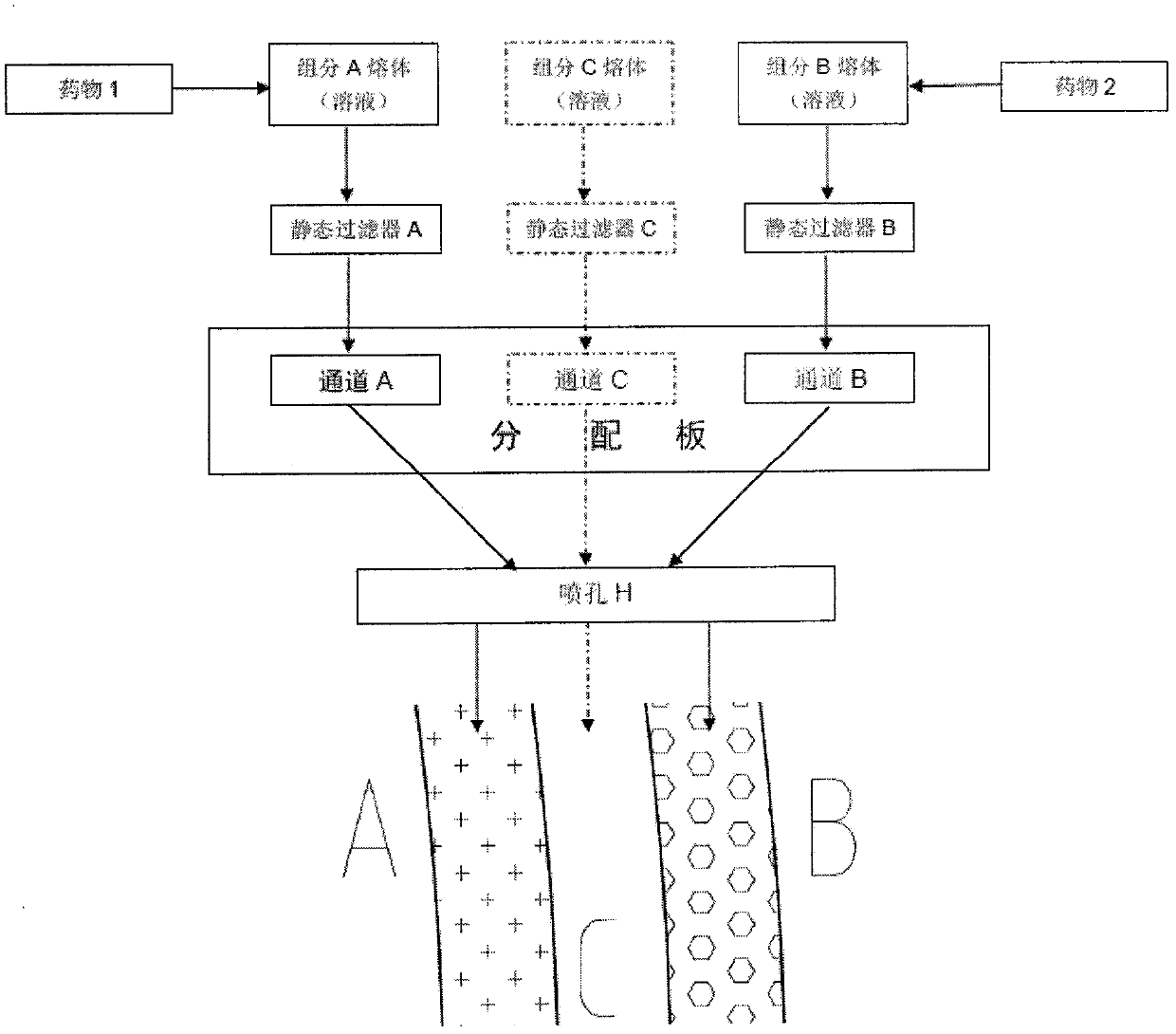
[0052] Melt 500g of component APCL and 500g of component BPCL on the screw respectively to prepare a melt. While preparing the melt, uniformly add 10g of drug 1 cilostazol to component A, and add 10g of drug 2 rapamycin into B component. The component A PCL melt of completely miscible cilostazol and the component B PCL melt of completely miscible rapamycin were further mixed uniformly through two static filters respectively.
[0053] Component A PCL melt containing cilostazol enters channel A of the distribution plate, and component B PCL melt containing rapamycin enters channel B of the distribution plate.
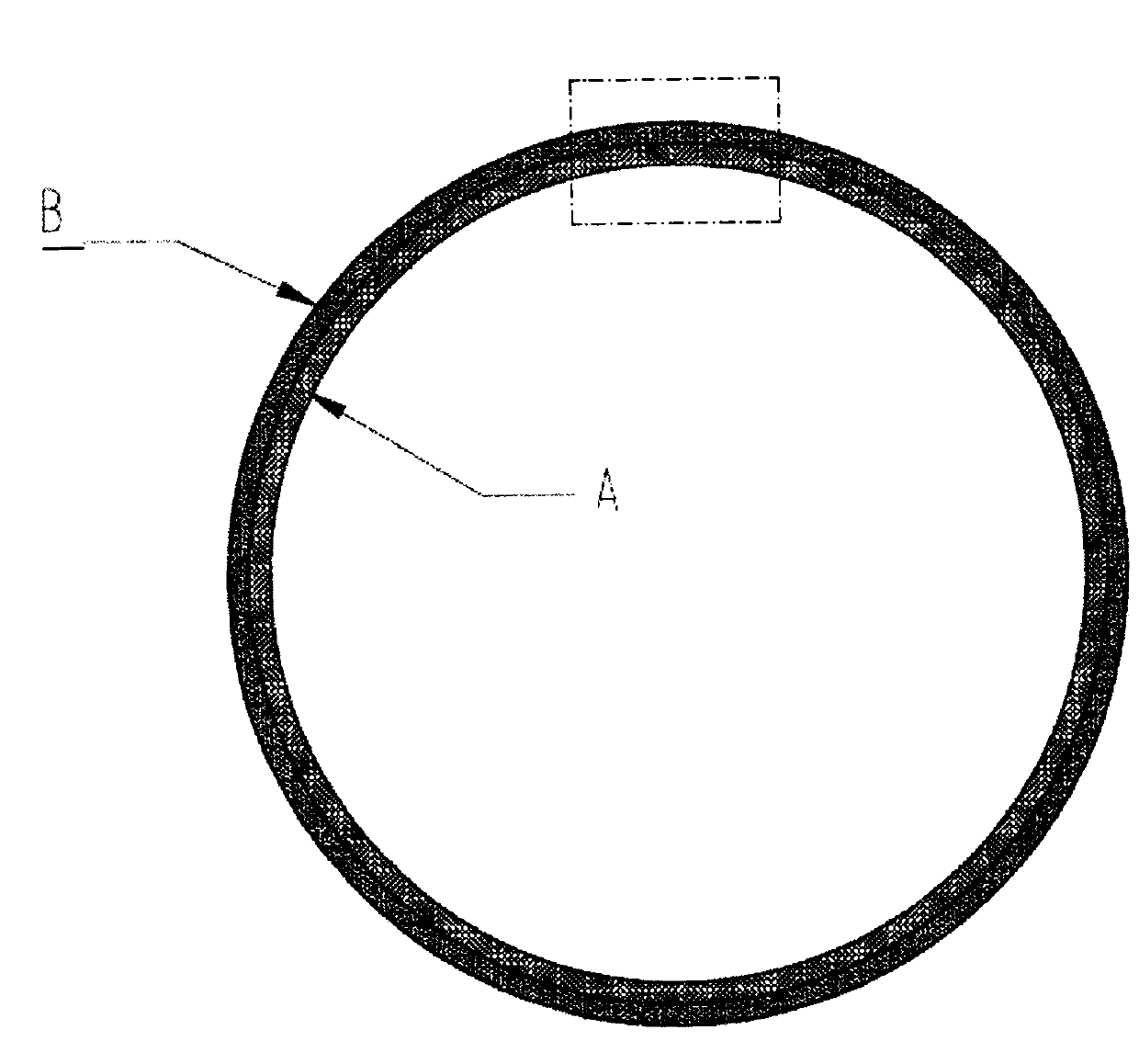
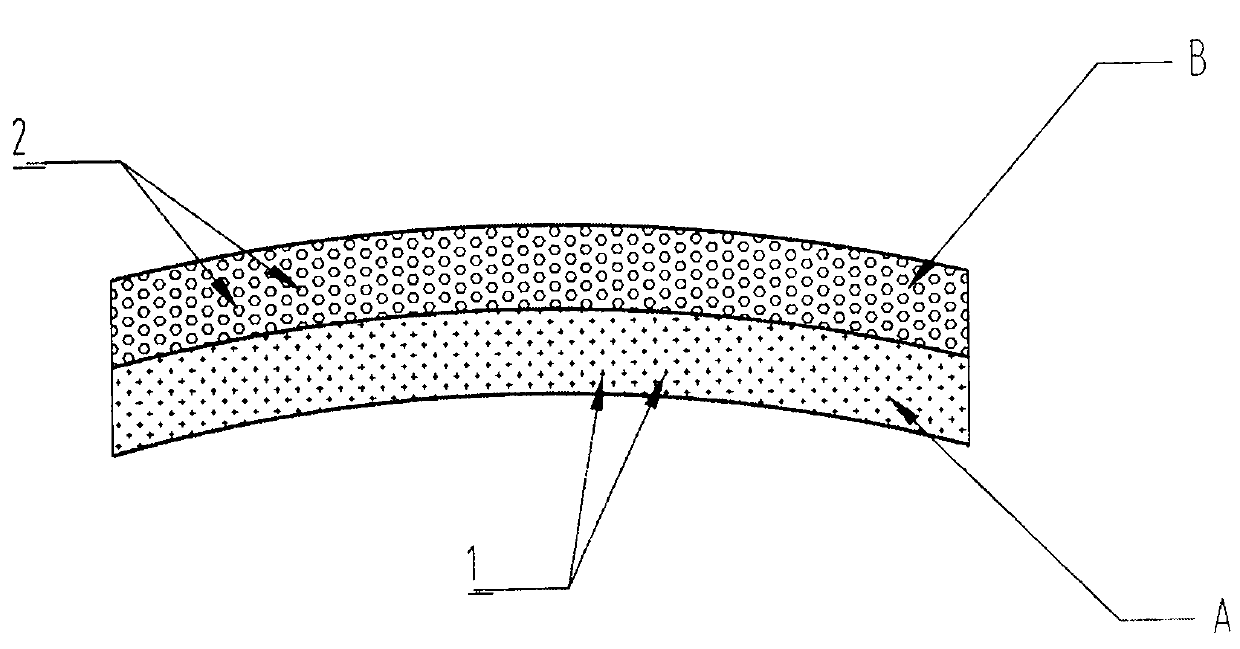
[0054] The component A PCL melt containing cilostazol and the component B PCL melt containing rapamycin are ejected together from the nozzle H under pressure, and the component containing cilostazol is ejected at the same time The A PCL melt was completely coated in...
Embodiment 2
[0057] Component A: PLGA500g Component B: PLGA500g Component C: PLLA1000g
[0058] Drug 1: Cilostazol 10g Drug 2: Rapamycin 10g
[0059] 500g of Component A PLGA, 500g of Component B PLGA and 1000g of Component C PLLA were respectively added to a sufficient amount of chloroform to prepare a polymer solution. While the solution was being prepared, 10g of drug 1 cilostazol was evenly added to the For component A, add 10 g of drug 2 rapamycin to component B. Component A PLGA solution that is completely miscible with cilostazol, component B PLGA solution that is completely miscible with rapamycin, and component C PLLA solution are further mixed uniformly through three static filters respectively.
[0060] Component A PLGA solution containing cilostazol enters channel A of the distribution plate, component B PLGA solution containing rapamycin enters channel B of the distribution plate, and component C PLLA solution enters channel C of the distribution plate.
[0061] The componen...
Embodiment 3
[0065] Component A: 500g of PLLA+PDLLA mixture Component B: 500g of PLLA+PDLLA mixture
[0066] Component C: PLLA2000g
[0067] Drug 1: Probucol 10g Drug 2: Paclitaxel 10g
[0068] 500g component A PLLA+PDLLA mixture and 500g component B PLLA+PDLLA mixture and 2000g PLLA were melted on the screw respectively to prepare a melt. While the melt was being prepared, 10g of drug 1 probucol was evenly added to component A, Add 10 g of drug 2 paclitaxel to component B. The melt of component A PLLA+PDLLA mixture of probucol completely mixed and the melt of component B PLLA+PDLLA mixture of paclitaxel and the melt of component C PLLA were respectively passed through three
[0069] Static filter for further mixing.
[0070] Component A PLLA+PDLLA mixture melt containing probucol enters channel A of the distribution plate, component B PLLA+PDLLA mixture melt containing paclitaxel enters channel B of the distribution plate, and component C PLLA melt enters channel C of the distribution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com