Suppository for treating mammal endometritis
An endometritis, mammalian technology, applied in the directions of suppository delivery, drug combination, medical preparations with inactive ingredients, etc., can solve the problems of small distribution area of drugs in the uterus, unfavorable infiltration into the uterine mucosa, and effects on uterine recovery. , to achieve non-toxic side effects, overcome the effects of irritating, strong bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
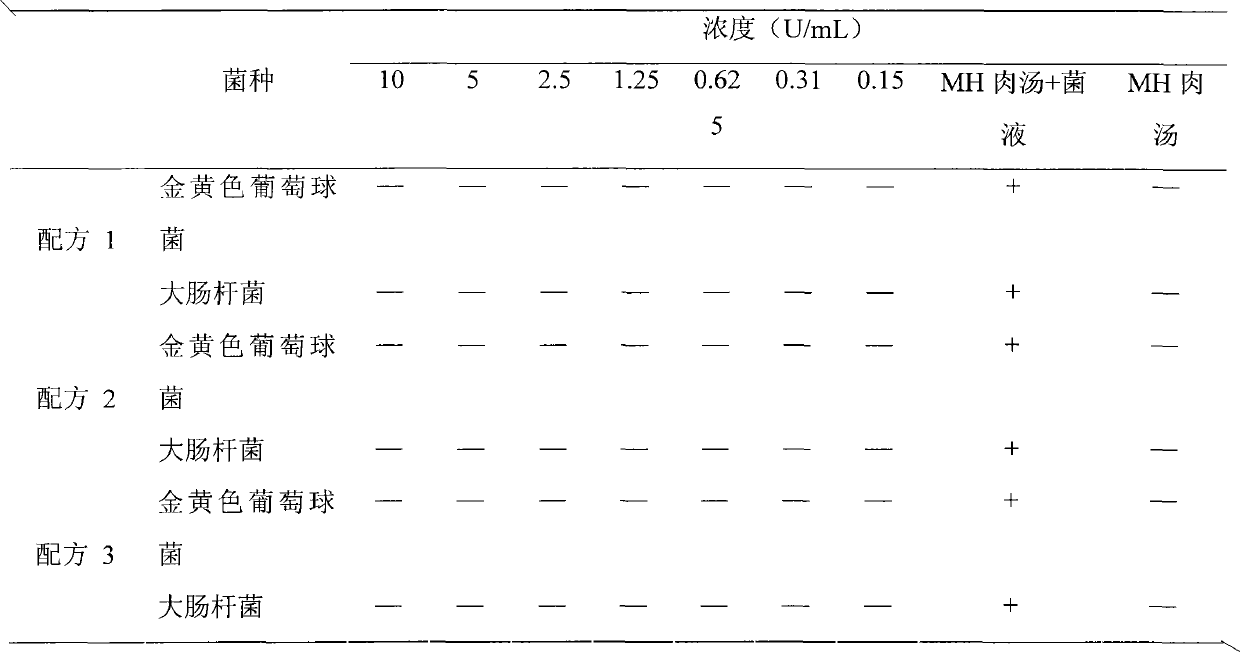
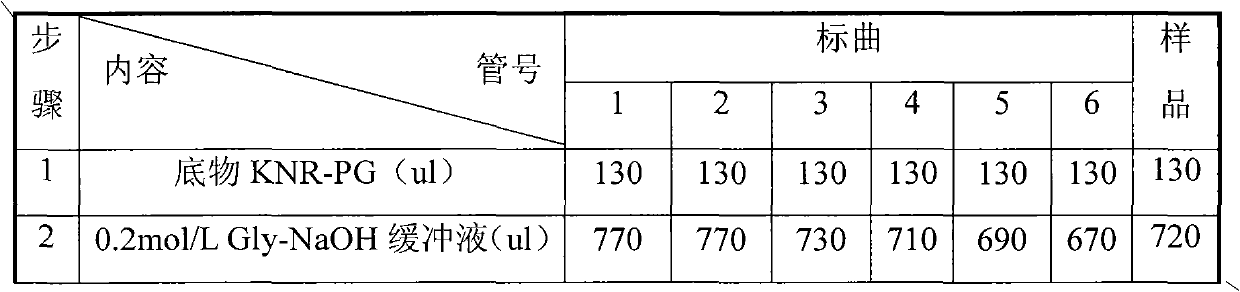
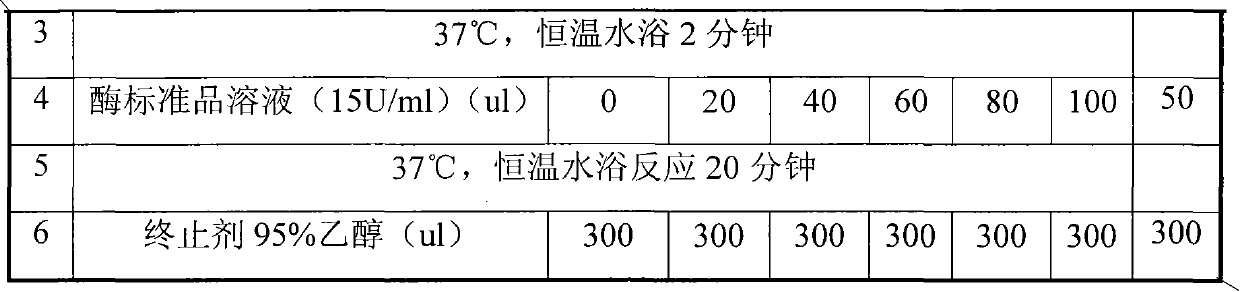
[0028] Embodiment 1 Composite enzyme suppository preparation method 1 (prescription 1)
[0029] 1. Composition and ratio
[0030] Lysostaphin 0.007%, lysozyme 0.1%, F6820%, HPMC 10%, sodium bicarbonate 10%, sodium dihydrogen phosphate 30%, semi-synthetic fatty acid glyceride 29.893%.
[0031] 2. Preparation
[0032]Weigh 0.007 g of lysostaphin, 0.1 g of lysozyme, 20 g of F68, 10 g of HPMC, 10 g% of sodium bicarbonate, and 30 g of sodium dihydrogen phosphate. The above raw materials are crushed through a 80-mesh sieve, mixed evenly, evenly mixed with 29.893 g of molten semi-synthetic fatty acid glycerides, and quickly poured into suppository molds to form.
Embodiment 2
[0033] Embodiment 2 Composite enzyme suppository preparation method 2 (prescription 2)
[0034] 1. Composition and ratio
[0035] Lysostaphin 0.004%, lysozyme 2%, F6810%, HPMC 20%, sodium bicarbonate 20%, sodium dihydrogen phosphate 10%, semi-synthetic fatty acid glyceride 37.996%.
[0036] 2. Preparation
[0037] Weigh 0.004 g of lysostaphin, 2 g of lysozyme, 10 g of F68, 20 g of HPMC, 20 g% of sodium bicarbonate, and 10 g of sodium dihydrogen phosphate. The above raw materials are crushed through an 80-mesh sieve, mixed evenly, evenly mixed with 37.996 g of molten semi-synthetic fatty acid glycerides, and quickly poured into a suppository mold to form.
Embodiment 3
[0038] Embodiment 3 Composite enzyme suppository preparation method 3 (prescription 3)
[0039] 1. Composition and ratio
[0040] Lysostaphin 0.01%, lysozyme 4%, F681%, HPMC 1%, sodium bicarbonate 30%, sodium dihydrogen phosphate 20%, semi-synthetic fatty acid glyceride 43.99%.
[0041] 2. Preparation
[0042] Weigh 0.01 g of lysostaphin, 4 g of lysozyme, F681 g, 1 g of HPMC, 30 g% of sodium bicarbonate, and 20 g of sodium dihydrogen phosphate. The above raw materials are crushed through an 80-mesh sieve, mixed evenly, evenly mixed with 43.99 g of molten semi-synthetic fatty acid glyceride, and quickly poured into a suppository mold to form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com