Gibberellin effervescent granules or tablets and preparation method thereof
A technology of effervescent granules and effervescent tablets, which is applied in the fields of botanical equipment and methods, biocides, animal repellants, etc., can solve the problems of inaccurate use of measurement, small powder particles, inaccurate measurement, etc. The effects of drug injury, rapid disintegration and accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
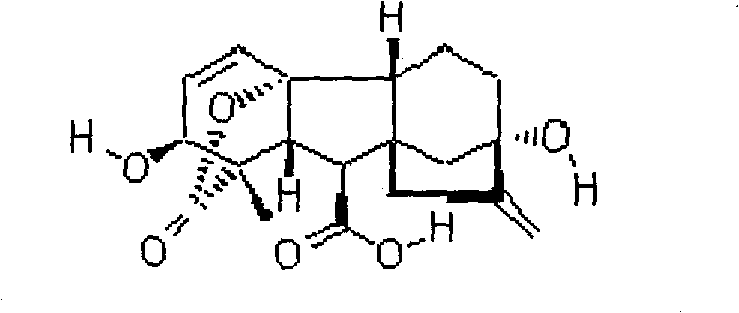
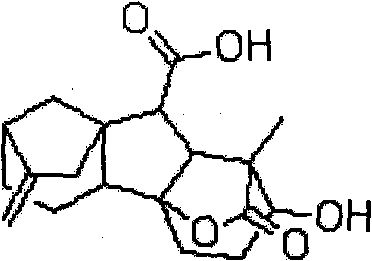
[0050] Example 1 0.1% gibberellin (GA 3 ) preparation of effervescent granules
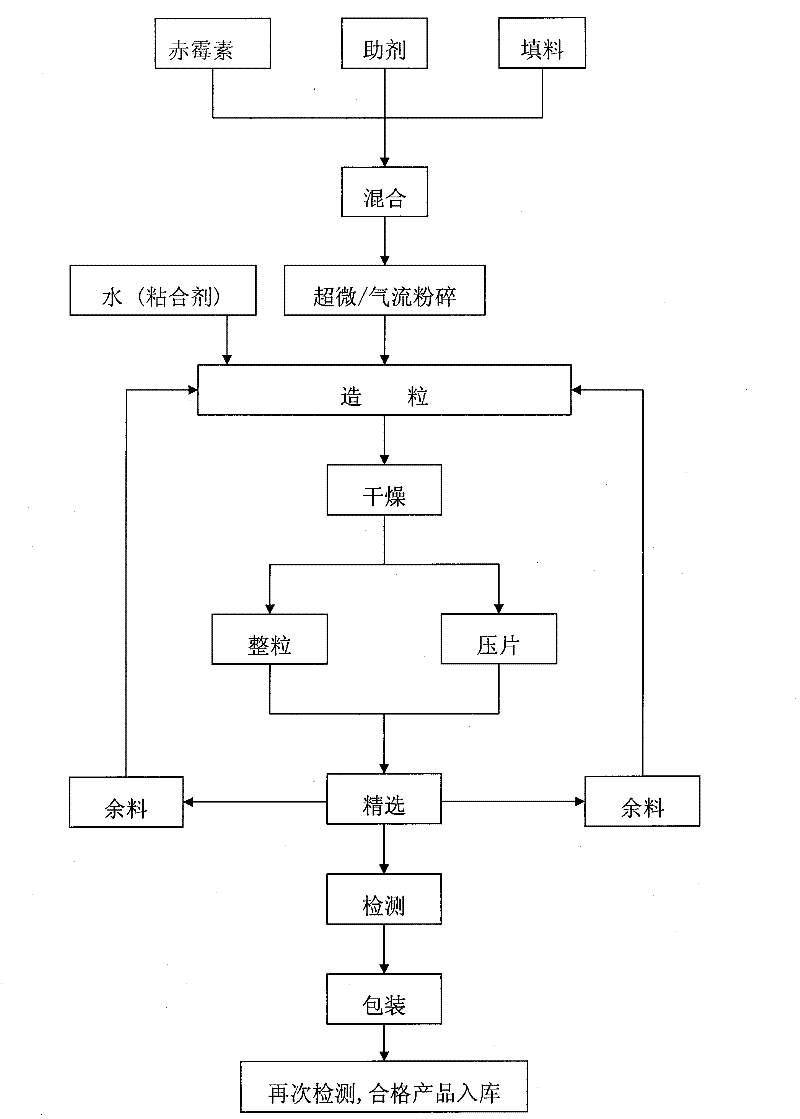
[0051] The selection and ratio of original drug and auxiliary agent are: 95% gibberellin (GA 3 ) 0.106% of the original drug (0.1%), the dispersant is 2.0% sodium methylene bismethyl naphthalene sulfonate, 4.0% calcium lignosulfonate, 2.0% wetting agent sodium lauryl sulfate, disintegration The agent is sodium bicarbonate 16.0%, sodium carbonate 4.0%, citric acid 15.0%, the binder is dextrin 5.0%, and the filler is attapulgite to make up 100%.
[0052] According to the above proportions, the dosage of each component is calculated, weighed, and mixed evenly; the formula is granulated by ultrafine / jet milling, dried, granulated, and selected. Product testing is carried out according to the quality control indicators of effervescent granules, including the content of active ingredients, wetting time, disintegration rate, foaming property, sieve analysis, shedding rate, pH range and thermal storage ...
Embodiment 2
[0059] Example 2 0.1% gibberellin (GA 3 ) preparation of effervescent tablet
[0060] The selection and ratio of original drug and auxiliary agent are: 95% gibberellin (GA 3 ) original drug 0.106% (0.1%), dispersant is naphthalene sulfonate formaldehyde condensate 3.0%, calcium lignosulfonate 3.0%, wetting agent sodium lauryl sulfate 2.0%, disintegrant is carbonic acid Sodium hydrogen 21.0%, citric acid 17.0%, binding agent is polyvinyl alcohol 5.0%, filler is talcum powder and makes up 100%.
[0061] According to the above proportions, the dosage of each component is calculated, weighed, and mixed evenly; the formula is granulated by ultrafine / jet milling, dried, pressed into tablets, and selected. Product testing is carried out according to the quality control indicators of effervescent tablets, including the content of active ingredients, disintegration time limit, foaming property, sieve analysis, powder and fragment rate, pH range and thermal storage stability, etc. Th...
Embodiment 3
[0069] Example 3 1% gibberellin (GA 3 ) preparation of effervescent granules
[0070] The selection and ratio of original drug and auxiliary agent are: 95% gibberellin (GA 3 ) original drug 1.06% (1.0%), the dispersant is sodium methylene bismethylnaphthalene sulfonate 5.0%, the wetting agent is sodium dodecylbenzene sulfonate 3.0%, and the disintegrating agent is sodium carbonate 8.0% %, sodium bicarbonate 10%, citric acid 18%, binder is sodium carboxymethyl cellulose 3.0%, filler is kaolin to make up 100%. The specific preparation process is the same as in Example 1.
[0071] The gibberellin (GA) that embodiment 3 prepares 3 ) The assay method of each performance index of effervescent granule is the same as embodiment 1. The specific determination results are: gibberellin (GA 3 ) content 1.01%, wettability qualified (24 seconds), disintegration qualified (48 seconds), foaming property qualified (23 ml), moisture 1.0%, pH value 7.2, shedding rate 0.6%, sieve analysis 99%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com