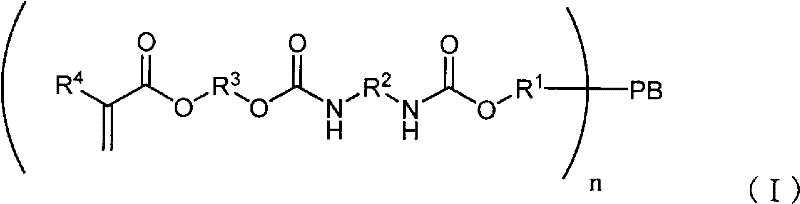
Method for producing polybutadiene
A technology of polybutadiene and manufacturing method, which is applied in the directions of conjugated diene adhesive, photoengraving process of pattern surface, adhesive type, etc., can solve problems such as unpredictability and reduction, and improve utilization value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] 212 g of n-hexane was placed in a 1 L flask, and cooled to -40°C. Add 23g of n-butyllithium n-hexane solution (concentration is 15.4% by mass), then add 48g of THF solution of tert-butoxide potassium (concentration of tert-butoxide potassium is 12.4% by mass, the amount added is the same as that of n-butyl Lithium equimolar), stirred at -40°C for 30 minutes. 14 g of butadiene liquefied at -78°C was added dropwise thereto, followed by stirring at -20°C for 1 hour. Further, 35 g of liquefied butadiene was added dropwise, followed by stirring at -20°C for 1 hour. Then 17 g of methanol were added.
[0115] This solution was washed twice with 200 g of pure water, three times with 200 g of 0.2% hydrochloric acid, further diluted with 180 g of toluene, and then washed four times with 300 g of pure water. The organic layer was concentrated and the solvent was distilled off to obtain 48 g of liquid resin.
[0116] The physical property values of the obtained resin are as f...
Embodiment 2
[0121] 278 g of THF was placed in a 1 L flask, and 2.7 g of a sodium dispersion (kerosene dispersion of metal sodium, concentration 43.7%) was added at room temperature, followed by cooling to -40°C. Next, 44 g of a THF solution of potassium tert-butoxide (the concentration of potassium tert-butoxide is 12.4% by mass, and the amount added is equimolar to sodium) was added, followed by stirring at -40° C. for 30 minutes. Continue to add 7 g of liquefied butadiene. After stirring at -20° C. for 1 hour, 42 g of liquefied butadiene were added. After further stirring at -20°C for 2 hours, 16 g of methanol was added.
[0122] This solution was poured into 1480 g of methanol, the supernatant liquid was removed by decantation, and the syrupy precipitate was dissolved in 162 g of toluene. This solution was washed once with 150 g of pure water, once with 150 g of 0.2% hydrochloric acid, and then three times with 300 g of pure water. The organic layer was concentrated and the solvent ...
Embodiment 3
[0127]196 g of THF was placed in a 1 L flask, and cooled to -40°C. Add 23g of n-butyllithium n-hexane solution (concentration is 15.4% by mass), then add 91g of THF solution of tert-butoxide potassium (concentration of tert-butoxide potassium is 12.4% by mass, the addition amount is n-butyl 2 times moles of lithium), stirred at -40°C for 30 minutes. 14 g of butadiene liquefied at -78°C was added dropwise thereto, followed by stirring at -20°C for 30 minutes. Further, 35 g of liquefied butadiene was added dropwise, followed by stirring at -20°C for 1 hour. Then 17 g of methanol were added.
[0128] This solution was washed once with 300 g of pure water, once with 200 g of 0.1% hydrochloric acid, and twice with 200 g of pure water. The organic layer was concentrated and the solvent was distilled off, and diluted with 90 g of THF. This solution was poured into 840 g of methanol, the supernatant liquid was removed by decantation, and the syrupy precipitate was dissolved with 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com