Yolk antibody for preventing and treating colibacillosis, preparation method for yolk antibody and feed additive
A technology for colibacillosis and yolk antibody, applied in the direction of antibodies, animal feed, animal feed, etc., can solve the problems of difficult vaccine and drug prevention, poor cross-immunization effect, etc., and achieve the effect of easy industrialization promotion and safety protection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
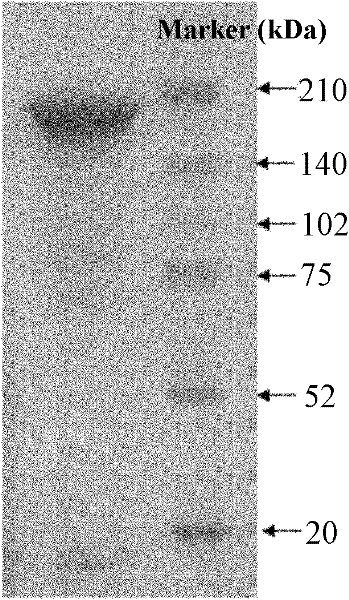
[0027] Embodiment 1 immunization uses the preparation of Escherichia coli outer membrane protein and pili protein mixed antigen and Escherichia coli egg yolk antibody
[0028] 1. Preparation of Escherichia coli outer membrane protein and pili protein mixed antigen for immunization
[0029] (1) Extraction of Escherichia coli outer membrane protein
[0030] avian pathogenic Escherichia coli O 78 Streak inoculation of strains on LB plates, culture at 37°C for 18 hours, pick a single colony, inoculate 3mL LB broth with shaking at 37°C for 15 hours, then inoculate 2mL LB broth into 200mL LB broth, continue to culture for 15 hours, take Centrifuge the bacterial solution at 6000g at 4°C for 10min, discard the supernatant, suspend the precipitate in 10mmol / L HEPES (pH7.4), ultrasonically lyse at 50% output power for 60s, centrifuge the lysate at 6000g at 4°C for 10min, collect the supernatant and add about 8 times the volume of 2% sodium lauryl sarcosinate solution, act at room temp...
Embodiment 2
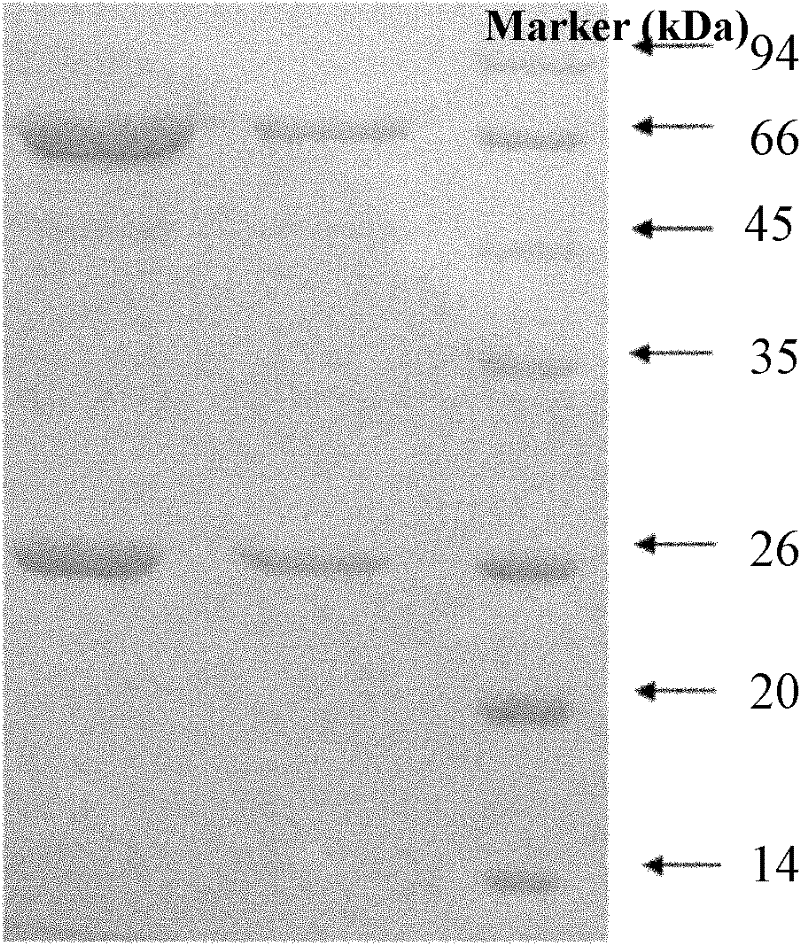
[0048] Example 2 Preparation of Escherichia coli outer membrane protein and pili protein mixed antigen and Escherichia coli egg yolk antibody for immunization
[0049] 1. Preparation of Escherichia coli outer membrane protein and pili protein mixed antigen for immunization
[0050] (1) Extraction of Escherichia coli outer membrane protein
[0051] avian pathogenic Escherichia coli O 78 Streak inoculation of strains on LB plates, culture at 37°C for 18 hours, pick a single colony, inoculate 3mL LB broth with shaking at 37°C for 15 hours, then inoculate 2mL LB broth into 200mL LB broth, continue to culture for 15 hours, take Centrifuge the bacterial solution at 6000g at 4°C for 10min, discard the supernatant, suspend the precipitate in 10mmol / L HEPES (pH7.4), ultrasonically lyse at 50% output power for 60s, centrifuge the lysate at 6000g at 4°C for 10min, collect the supernatant and add about 8 times the volume of 2% sodium lauryl sarcosinate solution, act at room temperature ...
Embodiment 3
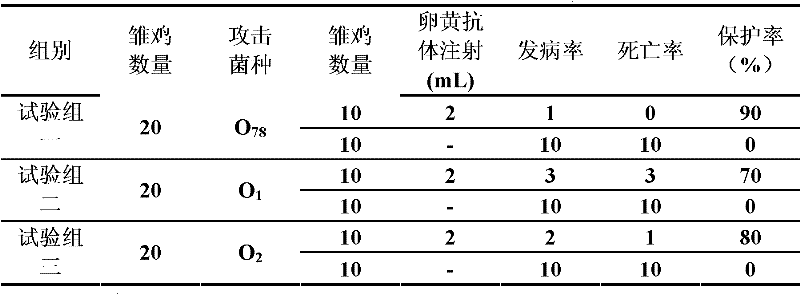
[0064] Example 3 Escherichia coli mixed egg yolk antibody protection test
[0065] Take 60 chicks aged 5-10 days for chick infection and treatment test, divide the chicks into 3 groups randomly, 20 in each group, the first group attacks avian pathogenic Escherichia coli O 78 , the second group challenged avian pathogenic E. coli O 1 , the third group challenged avian pathogenic E. coli O 2 , all adopt the way of subcutaneous injection in the neck, the dose is 5×10 7 CFU / only, 10 animals were randomly selected from each group 36 hours after the challenge, treated with the hyperimmune egg yolk antibody prepared in Example 1, each neck was subcutaneously injected with 2 mL egg yolk antibody, and the other 10 animals in each group were used for different treatments. As a treatment control, no reagent was injected, the death of each group of animals was observed, the death rate was calculated, and the therapeutic effect of the egg yolk antibody was evaluated.
[0066] As a resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com