Ursolic acid derivative and preparation method thereof
A technology of ursolic acid and derivatives, which is applied in the field of ursolic acid derivatives and can solve problems such as insignificant effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
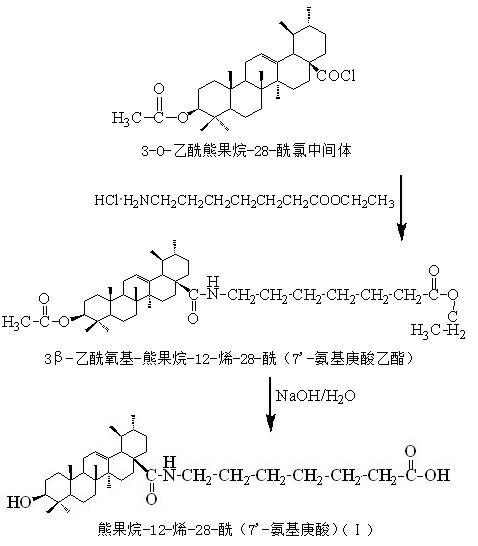
[0026] One, a kind of ursolic acid derivative, the parent in this derivative structure is ursolic acid, among the present invention, on the 28-position of ursolic acid parent, be connected with 28-acyl (7'-aminoheptanoic acid), The ursolic acid derivative is arbutane-12-ene-28-acyl (7'-aminoheptanoic acid), and its structural formula I is:
[0027] ;
[0028] Alternatively, there is 28-acyl (p-aminomethylbenzoic acid) at the 28-position of the parent ursolic acid, the ursolic acid derivative is ursolic-12-ene-28-yl (p-aminomethylbenzoic acid ), and its structural formula II is:
[0029] .
[0030] Two, a kind of preparation method of ursolic acid derivative, what this method prepared is the ursolic acid derivative described in this specific embodiment one; The method has following preparation steps:
[0031] (1) Reaction of ursolic acid and acetic anhydride to acetylate and protect the 3-hydroxyl of ursolic acid to generate 3-O-acetyl ursolic acid;
[0032] (2) React 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com