Transdermal granisetron
A skin and patch technology, applied in the field of transdermal patches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] To examine the possibility of incorporating granisetron into adhesives containing nucleophilic monomers, the drug was formulated into 4 different National Starch adhesives. As listed in Table 1, one of these adhesives contained a polymer with no functional groups, two contained polymers with acidic functional groups, and the fourth contained hydroxyl functional groups.
[0061] Table 1
[0062] Adhesive
chemical components
functional group
DT 4098
none
0
DT 2052
COOH
5
DT 2353
COOH
5
DT 2287
Acrylate-vinyl acrylate
Oh
5
[0063] "~% functional monomer" indicates the approximate level (w / w) of acidic or OH containing monomers used to make the adhesive, within 10%.
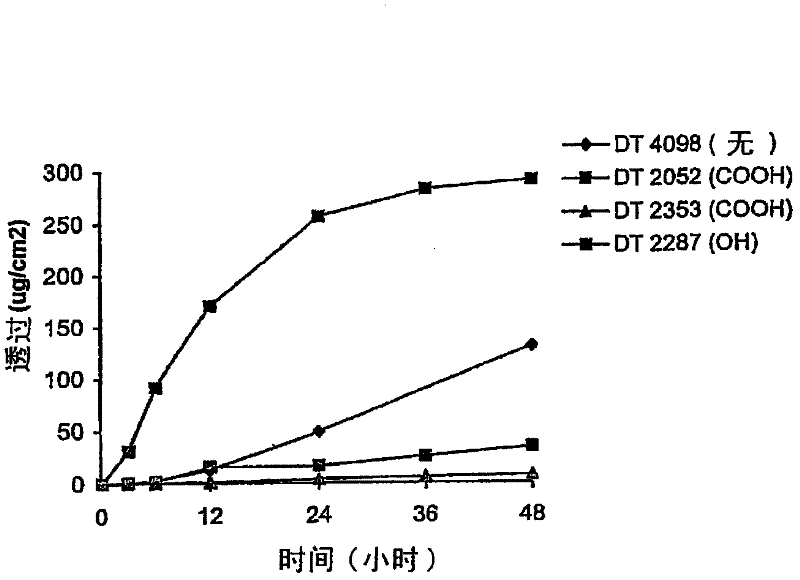
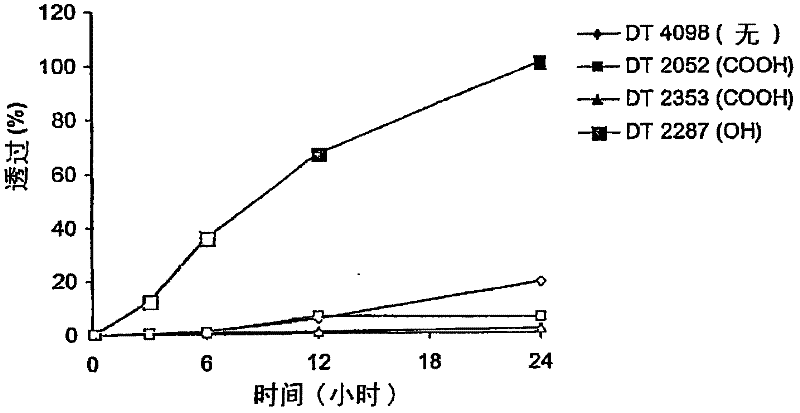
[0064] attached figure 1 The in vitro murine skin penetration of granisetron from formulations of 3% granisetron i...
Embodiment 2
[0078] Drug stability in the optimal formulation of Example 1 was investigated.
[0079] Stability data for patches formulated with DT 2287 and stored at three temperatures for 6 weeks are shown in Table 5. No decrease in granisetron content in the patches was observed, indicating that the drug remained stable in the three devices even under the accelerated conditions of 40 °C.
[0080] table 5
[0081] Stability of granisetron patch
[0082] Storage temperature
% granisetron
40℃
6 weeks
99.3
25℃
6 weeks
99.4
5℃
6 weeks
99.4
standard
99.5
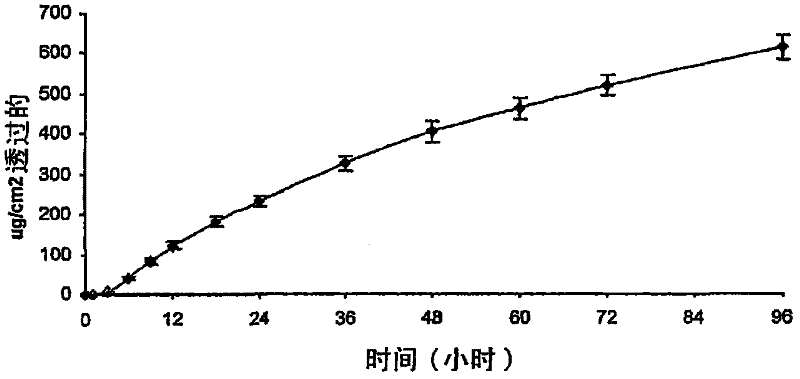
[0083] attached image 3 Indicates the in vitro human skin penetration of granisetron from DT 387-2287 adhesive (n=4), and depicts the use of 2 8% granisetron formulation for weight coating. This equates to about 880 μg / cm 2 granisetron loading. After 96 hours, the total permeation is about 600μg / cm 2 , which equals about 7...
Embodiment 3
[0088] Sustained delivery of granisetron in humans
[0089] Preparation of patch
[0090] Granisetron patches were prepared from the coating solution prepared as follows:
[0091] 1. Measure the solids content of DT 387-2287 and dilute the adhesive to the low specification limit of 49% with ethyl acetate. The solution was then stirred with a stirrer for 15 minutes.
[0092] 2. Dissolve granisetron base in dimethylacetamide (DMA) to obtain a clear solution (about 67 mg / ml).
[0093] 3. Mix the binder solution from 1 with the granisetron solution from 2.
[0094] The purpose of the coating process was to obtain a laminate with an areal weight of 110 g / sqm, low residual solvent levels and to limit the amount of drug degradation during the drying of the laminate.
[0095] The coating solution was coated on a release pad (FL2000100Fm) to obtain an area weight of 110 g / sqm. The backing foil Hostaphan MN 15MED was subsequently laminated. The production and stability of the coat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com