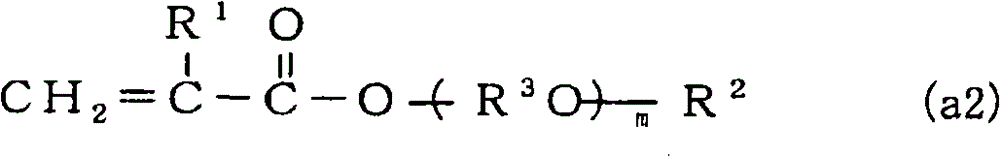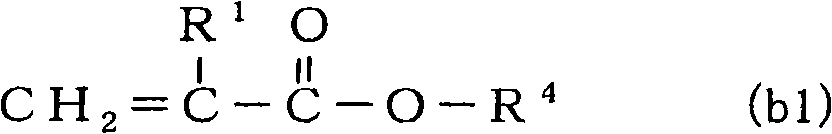Curable resin composition and cured product thereof
A curable resin and composition technology, applied in the direction of non-polymer organic compound adhesives, applications, coatings, etc., can solve the problems of resin composition strength, heat resistance reduction, insufficient terminal groups, etc., to achieve Effects of improved adhesion, excellent property balance, and high light transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0107] Next, although an Example demonstrates this invention, this invention is not limited to these Examples. It should be noted that the parts in each example are parts by weight. In addition, the measurement of each physical property in an Example carried out sample preparation and measurement by the method shown below.
[0108] 1) Molecular weight and molecular weight distribution of the polymer
[0109] The molecular weight and molecular weight distribution of the soluble polyfunctional (meth)acrylate copolymer were measured using GPC (Tosoh Corporation, HLC-8120GPC) in solvent: tetrahydrofuran (THF), flow rate: 1.0ml / min, column temperature: 40°C next. The molecular weight of the copolymer was measured as a polystyrene-equivalent molecular weight using a calibration curve derived from monodisperse polystyrene.
[0110] 2) The structure of the polymer
[0111] It was determined by 13C-NMR and 1H-NMR analysis and elemental analysis using a JNM-LA600 nuclear magnetic re...
Synthetic example 1
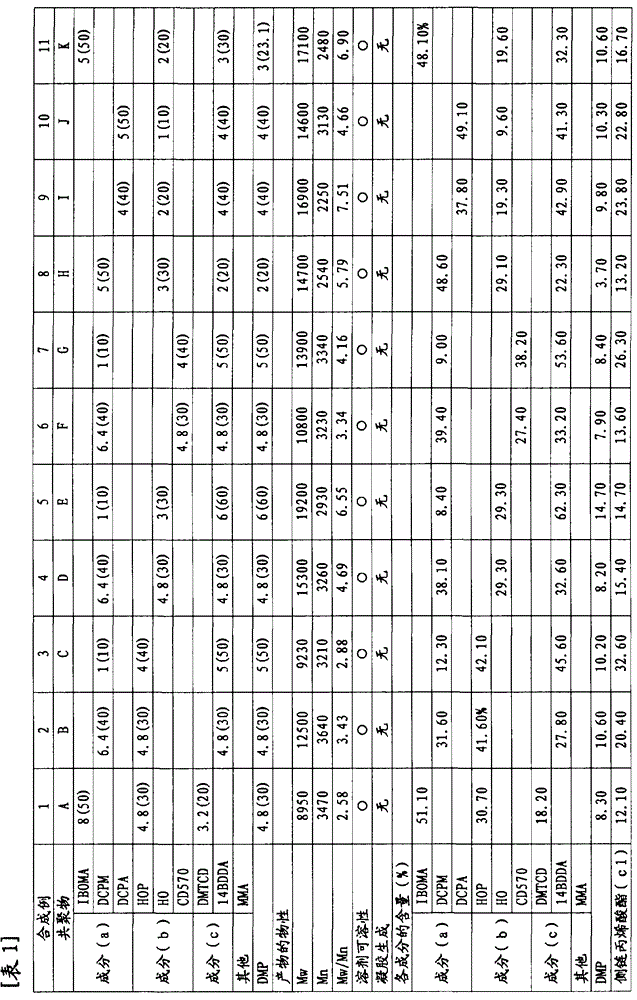
[0159] 3.2 moles (926.5ml) of dimethyloltricyclodecane diacrylate, 8.0 moles (1814.1ml) of isobornyl methacrylate, 4.8 moles (645.5ml) of 2-hydroxypropyl methacrylate, 2,4 - 4.8 moles (1145.9ml) of diphenyl-4-methyl-1-pentene and 2400ml of toluene were put into a 10.0L reactor, and 240mmol of benzoyl peroxide was added at 90°C, and allowed to react for 6 hours . After the polymerization reaction was stopped by cooling, the reaction liquid mixture was poured into a large amount of hexane at room temperature to precipitate a polymer. The obtained polymer was washed with hexane, filtered, dried, and weighed to obtain 1392.6 g of a copolymer A (yield: 40.5 wt %).
[0160] Mw of the obtained copolymer A was 8950, Mn was 3470, and Mw / Mn was 2.58. by carrying out 13 C-NMR, 1 H-NMR analysis and elemental analysis showed that copolymer A contained a total of 18.2 mol% of structural units derived from dimethyloltricyclodecane diacrylate, a total of 51.1 mol% of structural units deri...
Synthetic example 2
[0163] 4.8 moles (950.4g) of 1,4-butanediol diacrylate, 6.4 moles (922.7g) of tricyclo[5.2.1.02,6]dec-8-yl methacrylate, 2-hydroxypropyl methacrylate 4.8 moles (692.2g) of ester, 4.8 moles (1135g) of 2,4-diphenyl-4-methyl-1-pentene, and 2400ml of toluene were put into a 10.0L reactor, and 240mmol of supernatant was added at 90°C. Benzoyl was oxidized and allowed to react for 6 hours. After the polymerization reaction was stopped by cooling, the reaction liquid mixture was poured into a large amount of hexane at room temperature to precipitate a polymer. The obtained polymer was washed with hexane, filtered, dried, and weighed to obtain a copolymer B.
[0164] Mw of the obtained copolymer B was 12500, Mn was 3640, and Mw / Mn was 3.43. by carrying out 13 C-NMR, 1 H-NMR analysis and elemental analysis, copolymer B contains a total of 27.8 mol% of structural units derived from 1,4-butanediol diacrylate, and derived from tricyclo[5.2.1.02,6]dec-8-yl methacrylate A total of 27.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com