Method for preparing chiral alpha-alkyl substituted glycine hydrochloride
A glycine hydrochloride, hydrocarbyl substitution technology, applied in the field of synthesis of chiral amino acid hydrochloride, can solve the problems of high requirements, unstable optical purity of products, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
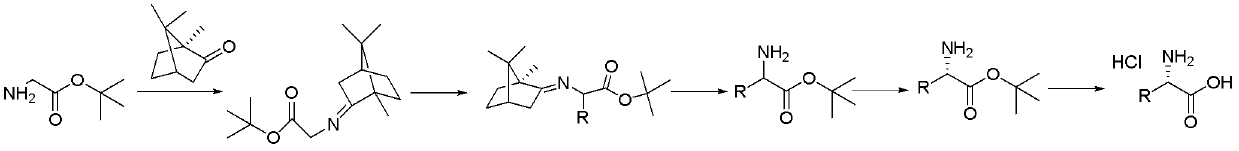
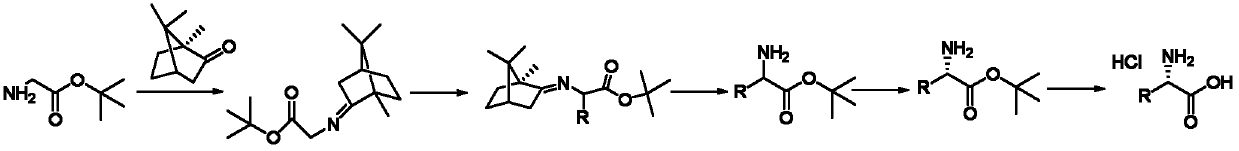
[0034] A kind of preparation L-allylglycine hydrochloride The method is characterized in that the specific preparation steps are as follows:
[0035] (1) Condensation: Add toluene 470kg (1g / 6mL), (S)-camphor 90kg (1.0eq), and main raw material glycine tert-butyl ester 116kg (1.5eq) to a 1000L reactor in sequence. After stirring evenly, add 1.26 kg boron trifluoride diethyl ether (0.015eq), after dropping, raise the temperature to 90±5°C for reaction; after the reaction, lower the temperature, wash the system with sodium bicarbonate solution until pH=7~8, and concentrate the organic phase to obtain the product 141kg, yield 90.0%, gas chromatography purity (GC): 96.8%;
[0036] (2) Substitution: Add 400kg of tetrahydrofuran (1g / 10mL) and 26.6kg (1.4eq) of potassium tert-butoxide into a 1000L reactor, cool down to -10±2°C, add dropwise 45kg of tetrahydrofuran (1g / 2mL) solution 125kg, after stirring for 0.5 hours, add 28.7kg (1.4eq) of 3-bromopropene dropwise, and react at -1...
Embodiment 2
[0042] A kind of preparation D-ethylglycine hydrochloride The method is characterized in that the specific preparation steps are as follows:
[0043] (1) Condensation: Add 66kg (1g / 1mL) of n-heptane, 100kg (1.0eq) of (S) camphor, and 86kg (1.0eq) of tert-butyl glycine as the main raw material into a 500L reactor. Boron trifluoride diethyl ether 932g (0.01eq), after dropping, raise the temperature to 80±2°C for reaction; after the reaction, cool down, wash the system with sodium bicarbonate solution until pH=7~8, and concentrate the organic phase to obtain the product 155kg, yield 89.0%, gas chromatography purity (GC) 96.5%;
[0044] (2) Substitution: Add 103kg (1g / 6mL) of 2-methyltetrahydrofuran (1g / 6mL) and 7.2kg (1.0eq) of sodium tert-butoxide into a 500L reaction kettle, cool down to -20-10°C, and dropwise add 20kg of 2-methyltetrahydrofuran (1g / 4mL) solution 90kg, after stirring for 0.3 hours, add 8.2kg (1.0eq) of bromoethane dropwise, and react at -20±2°C for 2 hours...
Embodiment 3
[0050] A kind of preparation L-benzylglycine hydrochloride The method is characterized in that the specific preparation steps are as follows:
[0051] (1) Condensation: 870kg (1g / 10mL) of xylene, 100kg (1.0eq) of (S) camphor, and 155kg (1.8eq) of tert-butyl glycine, the main raw material, were added in turn to a 2000L reactor. After stirring evenly, three Boron fluoride diethyl ether 1.86kg (0.02eq), after dropping, raise the temperature to 110±2°C for reaction; after the reaction, cool down, wash the system with sodium bicarbonate solution until pH=7~8, and concentrate the organic phase to obtain the product 160kg; yield 91.8%, gas chromatography purity (GC) 96.0%;
[0052] (2) Substitution: add 266kg methyl tert-butyl ether (1g / 12mL) and 28kg potassium carbonate (1.8eq) to a 1000L reactor, cool down to 10±2°C, add the main raw material dropwise 30kg of methyl tert-butyl ether (1g / 6mL) solution 164kg, after stirring for 1 hour, add 34.8kg of benzyl bromide (1.8eq) dropwi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com